Quality Systems and Compliance
PharmaLex can provide support with PQS planning, development and implementation. We can also remediate PQS that are not working properly and we can also provide support with the implementation or remediation of specific elements of the PQS such as Supplier management, Quality Risk Management, Change Management, Learning Management and deviation management to name a few. We can design and implement PQS for MIA, WDA and MAH.
Contact our specialists to tailor a service plan
Backbone of every life sciences organization
The Quality Management System (QMS) is the backbone of every life sciences organization. It is the formalized system that documents procedures, processes and responsibilities for achieving regulatory and quality policies and objectives.
A well designed, tailored and implemented QMS should enable and facilitate your organization to effectively and efficiently progress through R&D, Clinical and Commercial operations. A one-size-fits-all approach is neither practical nor preferable. Instead, the QMS should be phase-appropriate. It should be fitted and adapted to the organization’s development stage – whether you’re in early clinical, late clinical or commercial – and to your business model, for example: in-house manufacture versus virtual or contract manufacture.
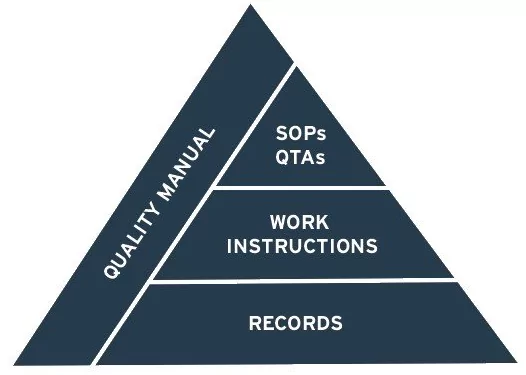
The QMS is now more commonly known as the Pharmaceutical Quality System as per ICH Q10. It contains several elements and process that are designed for the assurance of product quality. The design of the PQS is important to ensure that the system is holistic and synergistic as all elements must support and compliment each other to provide a quality backbone for the manufacturing of product. If the PQS is not appropriately planned, implemented and managed, it can become too complex with an ever increasing number of documents and leading to duplication and contradiction.
We offer global solutions covering the entire product lifecycle
Our solutions, consisting of core services, which are tailored to address each of your unique requirements. Extensive program management expertise is a key success factor within our service solutions.
Broad base of expertise
We have a broad base of expertise through our wide network of consultants, all of whom are SMEs in PQS planning, design and implementation. We can support CSV of computerized systems and have hands on experience with the implementation and comparison of several computerized systems that are used within the PQS. We also have automated tools for QRM. and we can provide case studies of successful PQS support for our clients across several different sectors from small businesses to large multinationals.
GxP Quality systems
The QMS contains several elements and processes that are designed for the assurance of product quality. The design of the QMS is important to ensure that the system is holistic and synergistic as all elements must support and complement each other to provide a quality backbone for the manufacturing of product. If the QMS is not appropriately planned, implemented and managed, it can become too complex with an ever-increasing number of documents and leading to duplication and contradiction.
PharmaLex provides support with QMS planning development and implementation. We can also remediate systems that are not working properly and provide support with the implementation or remediation of specific elements such as Supplier management, Quality Risk Management, Change Management, Learning Management and deviation management to name a few. We can design and implement Quality Management Systems for MIA, WDA and MAH
info
Before QMS development starts, it is critically important to understand the organisation in which the QMS will be implemented. This will determine how the core requirements will be developed, as well as identifying additional requirements to fit the organisation’s specific needs. This structure can broadly be broken into the following components:
We will guide you through the QMS design process, usually in a virtual or in-person workshop. Our consultants will then prepare a QMS Design report that presents you with a QMS framework and work plan for the development of the QMS. Where there is QMS documentation already available our philosophy is to leverage what’s available, update it where needed and develop new documents to fill the gaps.
QMS Development or Update
Whether it’s a resource, expertise or time constraint, PharmaLex is always ready and available to support your QMS development or QMS update. We have a broad base of expertise through our wide network of consultants, all of whom are SMEs in Quality Management system planning, design and implementation.
We can provide all development activities in full, including development / update of the Quality Manual / Site Master File, Standard Operating Procedures (SOPs), Forms, Quality Agreements, Training Material or Work Instructions.
We work in close association with your QA team throughout the development phase and we can flex up or down as your team needs support.
Contract QA/Virtual QA
PharmaLex has extensive experience in both the set up and operational support of quality and compliance services for different organisations which range from supporting a single element of the Quality Management System through to the outsourcing of the entire Quality department including external Quality oversight of Manufacturing and Supply Chain activities.
The range of services that PharmaLex support include, but are not limited to:
- Set up and implementation of CMO Onboarding processes
- Complaints, Recall and Product Quality Defects Management
- Staff augmentation (Qualified Person, Responsible Person, Quality Assurance Subject Matter Experts and Quality Assurance Operational staff)
- Vendor/Supplier Qualification and Management
- GxP Audits and Mock Inspections
Whether a company is seeking the setup of virtual quality and compliance activities or are seeking to outsource the quality and compliance element(s) of their business, our experienced experts have built up a wealth of expertise over the years and have a proven track record in line with our commitment to provide our clients “Confidence beyond Compliance”.
QP Services
Current legislation in Europe requires pharmaceutical companies to ensure that they engage a Qualified Person (QP) at every site that holds a Manufacturing or Importation Licence. Each batch of medicinal product released onto the European market must be dispositioned by a Qualified Person . The QP must also maintain oversight of the entire supply chain, provide QP Declaration where required and share responsibilities with other QPs in the supply chain if applicable.
PharmaLex experts include several QPs who are available to be named on our client’s European Manufacturer’s Importation Authorisation Licence (MIA) providing all QP services including:
- QA / QP support activities.
- Batch confirmation and disposition
- Management of the QMS
- Audit for QP Declaration
Available Resources
- 16th August 2023
FURTHER RESOURCES
Related Services
Commissioning, Qualification and Validation
We have been providing Commissioning, Qualification, and Validation (CQV) services to our clients for over 18 years. Our dedicated CQV professionals bring a depth of knowledge across the entire validation lifecycle (VLC), having supported clients from all major sectors within the Life Sciences industry. We guide our clients in the selection of the right CQV strategy and fully support all deliverables, from protocol preparation, through “on the floor executions” data analysis and Report preparation.
More InfoComputer System Validation (CSV)
Computer system validation provides documented evidence that software applications meet their intended uses by testing regulated functional processes as well as requirements related to electronic records and electronic signatures Contact our specialists to tailor a service plan Contact Us PharmaLex – powered by Arbour Group PharmaLex has merged with Arbour Group to deliver specialized software […]
More InfoGxP Audit
Ensuring your internal systems, or those of your supplier or client meet the necessary requirements, is essential before undertaking any regulatory process. It is also pertinent to follow good practice (GxP) guidelines in order to satisfy the regulators that all aspects meet their standards.
More InfoGxP Technical Consulting
Our GxP Technical Consulting Service provides access to our international Consulting Team, including a number of former EU and US Regulators, with expertise across the entire product lifecycle, ensuring that the broad Regulatory perspective is factored into the detailed consideration of your query
More InfoContact Us
Contact Form
Complete this form to stay in contact with us.
Global Approach
PharmaLex has 60+ offices in 32 countries serving more than 1600 satisfied clients worldwide. Search your nearest office.