CMC Services
CMC services strategy and documentation plays an important role from early development to authoring of the marketing authorization application dossier. It also extends to change control and maintenance throughout the product lifecycle. Moreover, CMC requirements are complex and different for chemicals, biologicals, biosimilars, herbals or homeopathics. PharmaLex guides you to the best CMC services for your products in line with health authority requirements and helps you to stay CMC compliant with sustainable processes.
Contact our specialists to tailor a service plan
IMPLEMENTING A SUSTAINABLE APPROACH TO THE CMC COMPLIANCE PROCESS
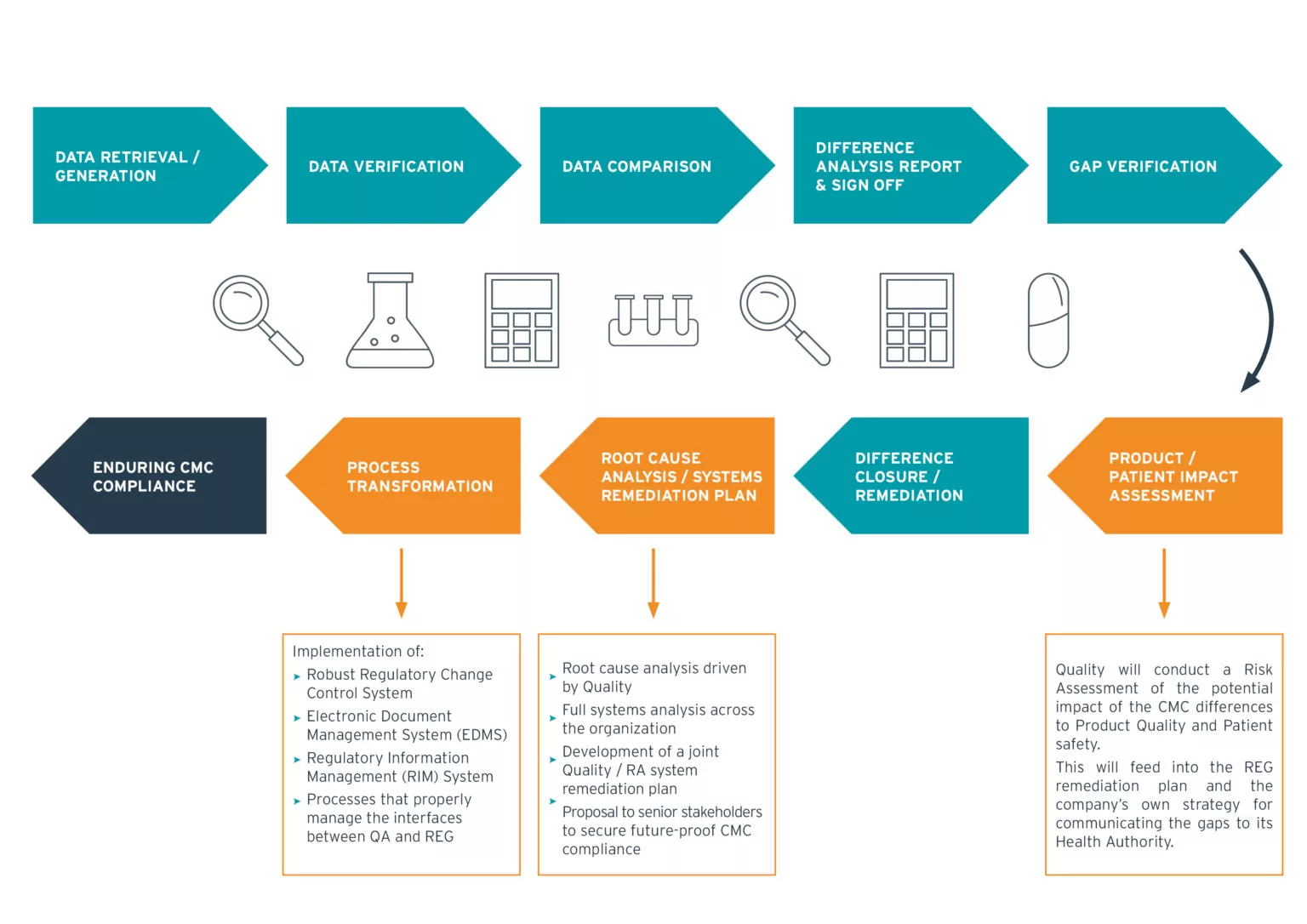
CMC (Chemistry, Manufacturing and Control) compliance is an important component in the lifecycle of a medicinal product, but is one that is continually challenged by many factors, including: Mergers & Acquisitions, increasingly complex networks of internal / external manufacturing sites and insufficiently robust internal processes to manage complexity
and cross- functional interfaces.
These factors often lead to compliance issues, which may have serious consequences from a delay in product approval requiring corrective actions up to product recalls and the loss of marketing authorizations.
We offer global solutions covering the entire product lifecycle
Our solutions, consisting of core services, which are tailored to address each of your unique requirements. Extensive program management expertise is a key success factor within our service solutions.
CMC content creation, compilation and maintenance support
Our services include:
- Product scope includes human and veterinary medicinal products comprising all types of APIs (chemicals, biologicals, biosimilars, herbals and homeopathics)
- Strategic consultancy during pharmaceutical development and lifecycle management
- Regulatory CMC intelligence and data management
- CMC scientific and technical writing
- Compilation of quality dossiers (ICH and non-ICH)
- CTD modules 3 and 2.3
- IMPD, IND
- ASMF / DMF, CEP
- Normative document
- CMC maintenance support: change control and quality variations
- GxP services (GMP, GACP, GDP) including audits
Our Chemistry, Manufacturing and Controls (CMC) team can provide you with regulatory strategy advice, scientific/technical writing and quality variation/amendment/supplement submission management on a global basis. We cover all CMC regulatory tasks from early development to authoring of the marketing authorization application dossier and beyond. We can give you regulatory guidance in dossier format, setting specifications, method development and validation documentation adapted to any type of medicinal product or drug substance/API and to any region worldwide. Whether it is a case of first-time submission, change control or maintenance throughout the product lifecycle, our multi-disciplinary team delivers CMC service that is aligned with the needs of your global submission management department, manufacturing sites and other stakeholders and that, of course, meets the latest Health Authority requirements.
CMC development biopharmaceuticals
The product types we support include:
- Recombinant proteins (e.g. mAbs (and derivatives), hormones, enzymes, fusion proteins)
- Tissue, cell and gene therapy products / ATMPs
- Biosimilars
- (Non-recombinant) biologicals isolated from living sources
- Peptides
- Antibody drug conjugates (ADC)
- Vaccines (recombinant proteins, live attenuates and recombinant vector vaccines)
Our services include:
- Development and regulatory strategy considering regional requirements (e.g. US, EU, Canada, Japan, Emerging Markets)
- Gap analysis / Due diligence
- Scientific and technical writing and compilation of regulatory documents
- Scientific advice and other Health Agency meetings
- Risk assessment
- Preparation of Target Product Profile (TPP)
- Comparability exercises
- General ‘troubleshooting’, including investigations and root cause analysis and support for CAPA
- Deviation and change control preparation / review
- Method / process validation
- Process characterization
- Project management
Biopharmaceuticals are an unprecedented success story. Due to their outstanding efficacy and safety and their high potential to address unmet medical needs, biopharmaceuticals are by far the fastest-growing part of the industry and are already making up 20 percent of the global pharma market.
But the effort and amount of technological skills required for the development and production of these complex molecules are both challenging and evolving rapidly, and so are the associated expectations of the regulators. Therefore, it is essential to account for regulatory requirements early on in development and to carve out the most efficient path to approval to ensure commercial success.
Based on a broad global project experience, we at PharmaLex recommend an integrated solution combining our scientific, technological and regulatory expertise to meet your specific support needs, both for specific aspects or for the complete development process.
CMC Compliance
Chemistry Manufacturing and Controls (CMC) compliance is an important component in the lifecycle of a medicinal product, but is one that is continually challenged by many factors, including Merger and Acquisition (M&A) programs, increasingly complicated networks of internal and external manufacturing sites and insufficiently robust internal processes within an organization to manage complexity and cross-functional interfaces.
These factors often lead to compliance issues, which may have serious consequences from a delay in product approval requiring corrective actions up to product recalls and the loss of marketing authorizations. It is clear that the impact of non-compliances identified through Health Authority inspections can be extremely costly and can irrevocably damage a company’s reputation.
A strong partnership is required between the Regulatory and Quality functions with the necessary inputs to support the implementation of transformed systems and processes within an organization, which can ultimately provide a sustained CMC compliance outcome for a business.
Our team of CMC and Quality experts will help you make your compliance proactive and in line with Health Agencies’ requirements, supporting the uninterrupted supply of medicines to patients. Besides broad experience, we bring a standardized approach and ready-to-use templates for compliance gap analysis and remediation to the table. And we can provide the needed resources to manage such projects even under high time constraints.
Available Resources
- 27th February 2025
FURTHER RESOURCES
Infographics
Related Services
Agency Interactions
PharmaLex has long-standing and extensive experience of interacting with the scientific committees and working parties of the FDA, EMA (e.g. CHMP, PRAC, COMP, PDCO, CAT) and with national Health Authorities. We frequently support our clients in Scientific Advice procedures at a national or central level, and in numerous health authority interactions throughout a product’s lifecycle (e.g. requests for PRIME eligibility, Orphan Medicinal Product designations, Pediatric Investigational Plans, Marketing Authorization Applications).
More InfoCMC Biologics
With over 75 CMC consultants across the globe, PharmaLex has substantial CMC expertise across all areas. We are able to offer an integrated solution combining the scientific, technological, non-clinical, clinical and regulatory expertise with our broad global project experience to meet your specific needs, from discovery to licensing and post-approval.
More InfoeCTD, System Operations and Data Management
Ensuring a smooth and effective submission process today goes beyond the old ways of eCTD publishing. For over 15 years, we have led the way in regulatory submissions with an evolving holistic approach that includes everything from planning to embedded quality control. Of course, all of our services are complemented by state-of-the-art digital facilitation.
More InfoPost-Approval Maintenance
Whether you are interested in outsourcing portfolio maintenance, require support during a merger and acquisition phase, need assistance covering your pharmacovigilance obligations or in development and implementation of your QMS—our Post-approval solutions provide trusted, strategic ways, as well as hands-on support, to keep your product compliant and moving forward.
More InfoPromotional Materials & Compliance
Pharmalex is your strategic partner to meet your goals for worldwide promotional material review and validation for drugs and medical devices. We provide a broad range of services linked to the review of promotional material for international congresses and local compliance. We will advise on and review activities and processes for healthcare compliance that fall […]
More InfoRegulatory Writing / Scientific and Technical Writing
PharmaLex offers scientific, regulatory and technical writing, supporting clients in the development of medicinal products.
More InfoContact Us
Contact Form
Complete this form to stay in contact with us.
Global Approach
PharmaLex has 60+ offices in 32 countries serving more than 1600 satisfied clients worldwide. Search your nearest office.