Monitoring and Signal Detection
PharmaLex customizes the monitoring and signal detection activities as per client requirements ensuring reliable, high quality, trackable data in compliance with the regulatory guidelines (e.g., GVP Module IX & FDA Good Pharmacovigilance Practice and Pharmacoepidemiologic Assessment).
In addition to Signal management services, our team of experts are also experienced and well versed in providing responses to safety questions from the Health authorities by providing strategic advice on the approach to respond to the HA query; evaluating and critically appraising ICSRs, aggregate data, and literature and thereby providing a comprehensive evaluation report considering the strengths and weaknesses of all the data sets and sources reviewed.
Contact our specialists to tailor a service plan
Managing all the steps of monitoring and signal detection
Signal detection at PharmaLex is performed by qualitative (review of ICSR, aggregate reports, literature) and quantitative methods (statistical analyses) or a combination of both. We review various sources of information including but not limited to ICSRs, literatures, Health authority websites (e.g., PRAC minutes & recommendations), EVDAS, FAERS, etc (This is primarily defined in conjunction with the client)
We ensure Signal detection methods applied are appropriate for the data set under analyses as qualitative analyses alone may not suffice for large data sets and complex statistical tools may not be appropriate for smaller data sets.
Focus areas:
- Information from all the available sources is reviewed and considered.
- Systems and SOPs in place to ensure consistent quality.
- Data is reviewed by a qualified person periodically.
- Audit trail with adequate documentation is maintained for the processes, procedures, and the systems for signal detection.
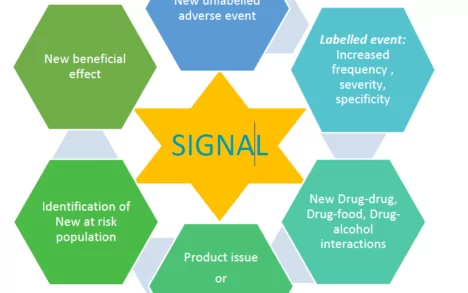
Identification of a signal does not mean that a drug has caused the adverse event. Evaluation of the signals is required to establish causality between the drug and the adverse event. Signal evaluation is a mandatory requirement to ensure that the benefit-risk balance of a drug is maintained, and it is a part of routine pharmacovigilance. Identification of a signal should prompt for further evaluation to classify the association.
Signal validation at PharmaLex is performed by:
- The supporting data of the detected signal is evaluated with an aim to verify that the available evidence contains sufficient evidence indicating a potentially new causal association or a new aspect of a known association justifying a further analysis of the signal.
- As a principle, only newly identified signals or known associations with new information on increased frequency, severity or occurrence in special populations are validated.
Factors considered while validating a signal:
a. Clinical relevance
- Strength of evidence supporting the causal association which depends on the number of reports, biological plausible mechanism, temporal relation, de-challenge, re-challenge, and exposure
- Severity, seriousness and outcome of the reaction
- Drug-drug interaction, drug-food interactions
- Novelty of the reaction-newly observed serious reactions
- Reactions observed in the special populations such as elderly, paediatric, pregnant and lactating mothers, hepatic or renal impaired patients, or populations not studied in the clinical trials
b. Previous awareness
- The available information in the product labels or patient leaflets
- Whether the signal was subject to regulatory request, discussed and accepted or refuted by scientific committees or if the association was assessed in PSUR or the RMP
c. Other Relevant Sources of Information
- Literatures with similar cases
- Pre-clinical or clinical studies on biological mechanisms
- Review of larger databases such as EudraVigilance, Vigibase, and FDA AERS for similar cases
In the event of insufficient information to validate a signal, cases with the event of interest are followed up for further information or conduct descriptive or analytical studies to reinforce the information to have a further understanding on the magnitude and clinical significance.
Prioritisation
While a signal is being validated by reviewing the evidence, in parallel the impact of the new safety finding on the public health, benefit-risk for the population being treated is promptly evaluated through Prioritisation. Prioritisation of a signal depends on multiple factors which may have an impact on the individual patient or a population.
Factors that are considered for prioritisation:
- New adverse drug reaction observed for the first time (Novel reactions)
- Seriousness and medical significance based on the severity, reversibility, morbidity, mortality and if the event is a Designated Medical Event (an event with high propensity to occur with drug administration and are serious with high mortality rate)
- Public health impact (is any alternate medication available for the indication the drug is used, impact of withdrawing the drug on the medical needs of the patients, etc.)
- Is there a recent increase in the disproportionality scores?
- Is there a temporal clustering of events?
- Are these events observed in special populations (paediatric, elderly, pregnant/lactating women, economic downtrodden, etc.)?
- Is it a recently launched drug?
- Risk perception and media attention
- Evidence from multiple sources and geographical locations
- Evaluate the impact depending on the severity, reversibility, clinical outcome, and the potential to prevent the event.
- Does the association reflect a clinical syndrome which includes other reactions in addition to the signal under study?
- Recommendation of time frame for the signal management should be the outcome of signal prioritisation. These outcomes should be entered in a signal tracking system along with the priority attributed and the justification.
The validated signals are comprehensively evaluated by PharmaLexperts to identify if any additional data can be collected to support the causal association and for any regulatory actions (e.g., CCDS, SmPC, RMP update etc).
Consistency of information among the various sources of information increases the likelihood of the causality for the association between the drug and the event. Information gathering to confirm, validate and recommend any action may take time. However, every effort is made to ensure the risks are prevented or minimised at the earliest.
Additional sources that PharmaLexperts look at:
- Pharmacokinetics and pharmacodynamics of the drug
- Pre-clinical study data on toxicology
- Clinical data
- Application dossier
- Expert consultation
- Epidemiological data
- Literature
- ICSR database
- Class effect analysis
- Search at MedDRA broad/narrow SMQ level
- Clinical complications of the events of interest
- External databases of the regulatory agencies (FDA AERS, WHO Vigibase, EU EudraVigilance)
Once the signals are identified as risks, further characterization is made by acquiring additional information through:
- Post-authorisation safety studies (PASS)
- Clinical trials
- Analytical/observational epidemiological studies
- Setting up active registries
On November 22nd, 2017 the EMA launched the new EudraVigilance database (EVDAS) and gave Marketing Authorization Holders (MAHs) access to the system. MAHs are required to inform the EMA and national competent authorities of validated signals detected for their products. It is expected that the data from EVDAS is taken into consideration during the preparation of PSURs in order to complement and enhance the signal assessments and the conclusions for issues under close monitoring.
We at PharmaLex, have a team of experts who perform the EVDAS review for the products in the EVDAS pilot list by monitoring the Signals of Disproportionate Reporting (SDR) and significant Reporting Odds Ratio (ROR) as defined in the EVDAS manual.
In addition to the routine review of the products under the EVDAS pilot list, EVDAS review is also performed during the Evaluation/Assessment of the validated signals.
Based on the signal evaluation/assessment and considering the impact of the safety issue on the benefit-risk of the product, PharmaLex proposes an appropriate action in consultation with the client.
In close coordination with our Regulatory team of experts, PharmaLex even supports the communication and dissemination of necessary regulatory actions to the Health authorities, patients, healthcare professionals and other stake holders, as required. (e.g. Submission of Variations, DHPC, etc)
We offer global solutions covering the entire product lifecycle
Our solutions, consisting of core services, which are tailored to address each of your unique requirements. Extensive program management expertise is a key success factor within our service solutions.
Available Resources
- 3rd August 2021
FURTHER RESOURCES
Related Services
Clinical Trials Adverse Event Processing
Clinical trials play a crucial part in any drug development life cycle where it is key to gain information on a product’s safety profile. The PharmaLex international team of clinical safety experts, with large expierence in the management of clinical safety activities, effectively supports sponsors with Pharmacovigilance activities during clinical trial development programmes. PharmaLex designs […]
More InfoGap Analysis and Consulting
Place your Pharmacovigilance, Epidemiology, Risk management (PER) and Document Management activities in experienced hands. Whether you need an urgent solution for an unexpected problem or are seeking to outsource entire processes – our knowledgeable experts will be there for you no matter where you are. Expertise extends to Clinical document management (e.g. TMFs) and Regulatory document management (e.g. submissions, RIM, xEVMPD, IDMP) through one of the PharmaLex family of companies.
More InfoICSR Management
The collection and processing of ICSRs are the most basic and important aspects of Pharmacovigilance system. PharmaLex delivers end-to-end ICSR Management solutions through our experts and services. Contact our specialists to tailor a service plan Contact Us ICSR (Individual Safety Case Report) Management PharmaLex offer the full range of ICSR Management Services from collection, triage, […]
More InfoLiterature Surveillance
We offer a modular, end-to-end approach to literature surveillance for pharmacovigilance and our experts are flexible enough to offer a tailored solution as well accordingly to client’s requirement.
More InfoPharmacovigilance Audits
PharmaLex’s organization ensures global delivery of all PV audit services. We are the first-choice provider of PV audit services for many pharmaceutical companies around the world. At PharmaLex, auditors are highly qualified with necessary and long-standing expierence as well as strong cultural awareness and excellent communication skills to effectively conduct and/or participate in PV audit activities.
More InfoQualified Person for Pharmacovigilance (QPPV)
If required, PharmaLex can take on the tasks and responsibilities of the EU Qualified Person Responsible for Pharmacovigilance, or provide a deputy in times of absence or illness.
More InfoContact Us
Contact Form
Complete this form to stay in contact with us.
Global Approach
PharmaLex has 60+ offices in 32 countries serving more than 1600 satisfied clients worldwide. Search your nearest office.