Gap Analysis and Consulting
Outsourcing some or all aspects has become a standard part of drug development and marketing in the pharmaceutical industry. Identifying a specific customizable model that matches the needs and strategy of the organization, is an important first step in this process. PharmaLex offers various options with flexible staffing from short-term tactical support to long-term strategic partnerships.
Place your Pharmacovigilance, Epidemiology, and Risk management activities (PER) in experienced hands. Whether you need an urgent solution for an unexpected problem or are seeking to outsource entire PER processes – our knowledgeable experts will be there for you no matter where you are.
Contact our specialists to tailor a service plan
We provide support
- ICSR management, including collection, evaluation, processing, distribution and reporting
- Safety database hosting and support
- Literature monitoring
- Pharmacoepidemiology
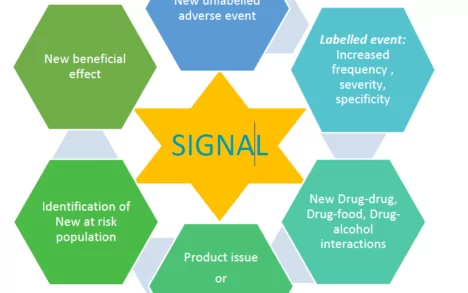
- Signal management
- Risk management
- Periodic safety reports
- Pharmacovigilance system and compliance
- EU-QPPV / local QPPV
- Quality system, audit and inspection support
- Safety data exchange agreements
We offer global solutions covering the entire product lifecycle
Our solutions, consisting of core services, which are tailored to address each of your unique requirements. Extensive program management expertise is a key success factor within our service solutions.
Available Resources
- 3rd August 2021
FURTHER RESOURCES
Related Services
Clinical Trials Adverse Event Processing
Clinical trials play a crucial part in any drug development life cycle where it is key to gain information on a product’s safety profile. The PharmaLex international team of clinical safety experts, with large expierence in the management of clinical safety activities, effectively supports sponsors with Pharmacovigilance activities during clinical trial development programmes. PharmaLex designs […]
More InfoICSR Management
The collection and processing of ICSRs are the most basic and important aspects of Pharmacovigilance system. PharmaLex delivers end-to-end ICSR Management solutions through our experts and services. Contact our specialists to tailor a service plan Contact Us ICSR (Individual Safety Case Report) Management PharmaLex offer the full range of ICSR Management Services from collection, triage, […]
More InfoLiterature Surveillance
We offer a modular, end-to-end approach to literature surveillance for pharmacovigilance and our experts are flexible enough to offer a tailored solution as well accordingly to client’s requirement.
More InfoMonitoring and Signal Detection
PharmaLex customizes the signal management activities as per client requirements ensuring reliable, high quality, trackable data in compliance with the regulatory guidelines (e.g., GVP Module IX & FDA Good Pharmacovigilance Practice and Pharmacoepidemiologic Assessment). In addition to Signal management services, our team of experts are also experienced and well versed in providing responses to safety questions from the Health authorities by providing strategic advice on the approach to respond to the HA query; evaluating and critically appraising ICSRs, aggregate data, and literature and thereby providing a comprehensive evaluation report considering the strengths and weaknesses of all the data sets and sources reviewed.
More InfoPharmacovigilance Audits
PharmaLex’s organization ensures global delivery of all PV audit services. We are the first-choice provider of PV audit services for many pharmaceutical companies around the world. At PharmaLex, auditors are highly qualified with necessary and long-standing expierence as well as strong cultural awareness and excellent communication skills to effectively conduct and/or participate in PV audit activities.
More InfoQualified Person for Pharmacovigilance (QPPV)
If required, PharmaLex can take on the tasks and responsibilities of the EU Qualified Person Responsible for Pharmacovigilance, or provide a deputy in times of absence or illness.
More InfoContact Us
Contact Form
Complete this form to stay in contact with us.
Global Approach
PharmaLex has 60+ offices in 32 countries serving more than 1600 satisfied clients worldwide. Search your nearest office.