By Eric Rozet, Director Statistics and Data Science
The new version of the ICH Q2(R2) document concerning the validation of analytical procedures has been released lately. It includes deeper modifications than its previous revision that may impact the validation strategy of the (bio)-pharmaceutical companies. This new version, written together with the companion ICH Q14 guidance on the development of analytical procedure, emphasis the importance of the life cycle of analytical procedure: putting back the robustness study to the development phase of the analytical procedure in coherence with the analytical Quality by Design idea and recalling the need to assess some validation performance characteristics when transferring an analytical procedure.
When looking specifically at the validation phase of ICHQ2(R2) document, here are some key additions to this guidance:
- The experimental design to assess the validation performance characteristics can be an “All-in-one” design: “In practice, the experimental work can be designed so that the appropriate performance characteristics are considered simultaneously to provide sound, overall knowledge of the performance of the analytical procedure” [ICH Q2(R2)].
- It makes the link between analytical procedure validation and the reportable result’s format, this makes it coherent with the USP-1033 Biological Assay Validation chapter where a detailed methodology to define a reportable result and it’s format is provided: “If justified, it may be acceptable to perform some validation tests using a different number of replicates or to adjust the number of replicates in the analytical procedure based on data generated during validation” [ICH Q2(R2)].
- For non-linear response analytical procedures and multivariate calibration ones ICHQ2(R2) specifically states that demonstration of the linearity of the response is not needed but rather the linearity of the results: see for e.g. “analytical procedure performance should be evaluated across a given range to obtain values that are proportional to the true (known or theoretical) sample values” [ICH Q2(R2)].
- For Accuracy and Precision, two approaches are suggested: either an individual evaluation of these two performance characteristics, as historically, or a combined evaluation, a modern approach.
- In the separate evaluation of Accuracy and Precision ICH Q2(R2) states that “…an appropriate 100(1-α) % confidence interval (or justified alternative statistical interval) should be reported.” And it further requires that the observed interval should be compatible with the corresponding accuracy or precision acceptance criteria. This suggest that the era of using the simple approach of comparing the estimated Recovery or the estimated CV to an acceptance criterion is going to be replaced in favor of equivalence testing such as suggested in USP-1210 Statistical Tools for Procedure Validation chapter.
- If one chooses to go for the combined approach for accuracy and precision, which is in fact called Total Error or Total Analytical Error [Hubert et al., 2004 & 2007], then only a single acceptance criterion is used and here again statistical intervals have to be used: “…Combined accuracy and precision can be evaluated by use of a prediction interval, a 07tolerance interval …” [ICH Q2(R2)]. Detailed statistical explanations can be found in USP 1210 chapter as well as in Hubert et al. 2007.
The aim of validation of analytical procedure is “to demonstrate that the analytical procedure is fit for the intended purpose”. The purpose of an analytical procedure is to provide an analytical result, or reportable result that can be trusted. With these modifications, ICH Q2(R2) focuses more on this global aim and our validation software Enoval is fully in line with it.
Enoval current version allows fulfilling all the requirements stated in ICHQ2(R2) about Accuracy, Precision (repeatability and Intermediate Precision), individually and combined, Linearity, Working Range, Lower limit of quantitation. In coherence with ICH Q14 and the analytical QbD paradigm highlighted in USP 1220, the Analytical Target Profile (ATP) can be included in the report. Requested recommended data are provided: correlation and determination coefficient, models equation of calibration curves, slopes and intercepts for results linearity, adequate residuals graphs, recoveries and their corresponding confidence intervals, CVs and their corresponding confidence intervals as well as Prediction intervals for the combined approach for accuracy and precision implementing the Total Analytical Error concept.
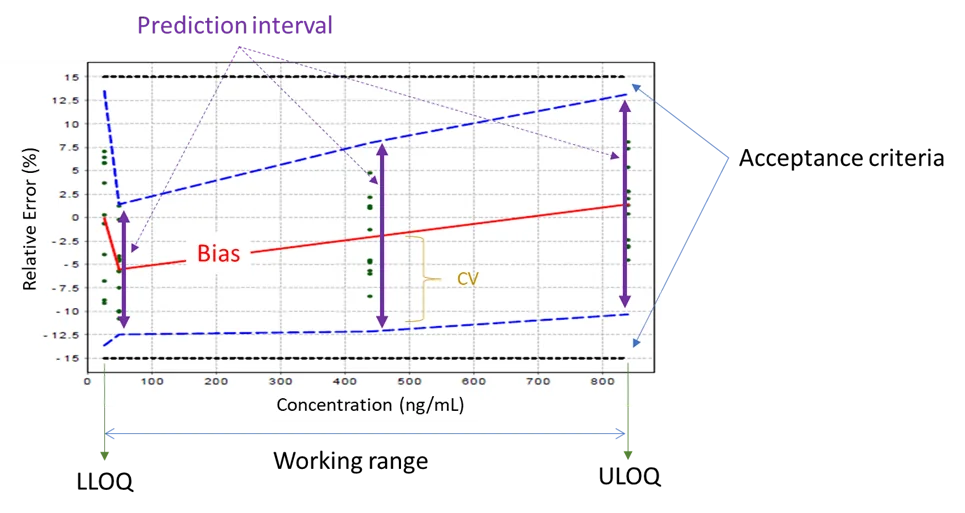
Using the combined approach or total analytical error, Enoval provides the Total Error graph to assess the validity of the analytical procedure in a glimpse, as shown in Figure 1.
Figure 1: Example of Total Error Profile of Enoval: As the blue dashed lines (95% Prediction intervals – Combined approach for Accuracy and Precision) are fully included into the black dotted lines (Acceptance limits for Total error set at +/-15%) the analytical procedure is valid from 20 ng/mL (Lower limit of quantitation – LLOQ) till 850 ng/mL (Upper limit of quantitation – ULOQ); hence the valid working range is 20 ng/mL – 850 ng/mL. (Note that the green dots are the analytical results)
Using Enoval, the process of validating analytical procedures following the new ICH-Q2(R2) guidance can be automated as well as provide a solution that:
- Is easy to use: made by statisticians for non-statisticians.
- Allows to Standardize procedure validation reporting across the organization.
- Is up to date with latest authority requirements (ICH-Q2(R2), EMA, FDA, …).
- Is suitable for GxP use (GAMP5 validated, 21 CRF Part 11 compliant).
- Is a SaaS: no maintenance costs (i.e., Cencora-Pharmalex maintains it for you).
- Reduces report writing and reviews to a couple of minutes instead of days.
- Goes further than what’s possible with Excel (e.g., REML, unbalanced designs, Prediction Intervals, etc.).
- Can be integrated into existing LIMS or data platforms.
References
ICH Q2(R2): Validation of Analytical Procedures (2023)
ICH Q14: Analytical Procedure Development (2023)
USP 1033: Biological Assay Validation
USP 1210: Statistical Tools for Procedure Validation
USP 1220: The Analytical Procedure Lifecycle
Hubert et al., Journal of Pharmaceutical and Biomedical Analysis 36 (2004) 579-586
Hubert et al., Journal of Pharmaceutical and Biomedical Analysis 45 (2007) 82–96