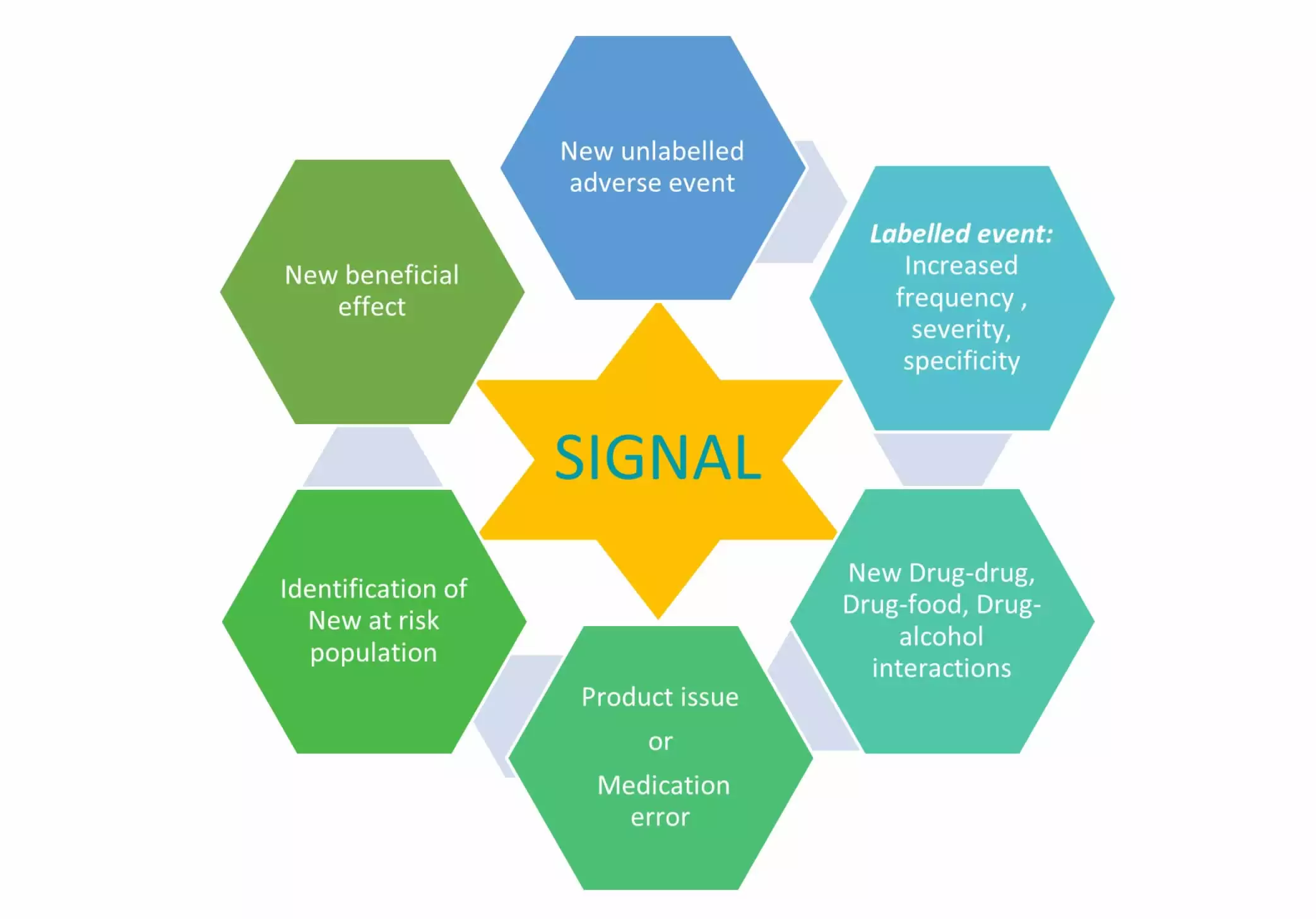
PharmaLex customizes the signal management activities as per client requirements ensuring reliable, high quality, trackable data in compliance with the regulatory guidelines (e.g., GVP Module IX & FDA Good Pharmacovigilance Practice and Pharmacoepidemiologic Assessment). Our experts can assist with continuous monitoring of the safety data to Detect, Validate, Prioritize, author Signal Assessment report, and Recommend Actions for signals through quantitative and qualitative reviews.
In addition to Signal management services, our team of experts are also experienced and well versed in providing responses to safety questions from the Health authorities by providing strategic advice on the approach to respond to the HA query; evaluating and critically appraising ICSRs, aggregate data, and literature and thereby providing a comprehensive evaluation report considering the strengths and weaknesses of all the data sets and sources reviewed.
On November 22nd, 2017 the EMA launched the new EudraVigilance database (EVDAS) and gave Marketing Authorization Holders (MAHs) access to the system. MAHs are required to inform the EMA and national competent authorities of validated signals detected for their products. It is expected that the data from EVDAS is taken into consideration during the preparation of PSURs in order to complement and enhance the signal assessments and the conclusions for issues under close monitoring.
We at PharmaLex, have a team of experts who perform the EVDAS review for the products in the EVDAS pilot list by monitoring the Signals of Disproportionate Reporting (SDR) and significant Reporting Odds Ratio (ROR) as defined in the EVDAS manual. In addition to the routine review of the products under the EVDAS pilot list, EVDAS review is also performed during the Evaluation/Assessment of the validated signals.
Our proprietary Macro based tool which reduces the efforts for EVDAS eRMR reports evaluation by around 50% by auto-populating the dispositions for drug-event pairs.
- Preparation and implementation of Signal monitoring plan
- Routine review of various sources for signals
- Validation and evaluation of signals
- Propose appropriate actions and assist in communicating and implementing these through PharmaLex Regulatory experts
- Strategic advice on responding to Health Authority queries on Safety issues
- Review of EVDAS eRMR reports through proprietary tool