Understanding and managing diverse pharmacovigilance (PV) requirements across different regions is a crucial element to a successful global expansion.
During a recent webinar, titled Navigating Local Pharmacovigilance Obligations in a Global Landscape, experts from several large and mid-sized pharmaceutical companies shared their experiences and insights on some of the key PV issues they and their colleagues at the regional and local level confront.
As companies expand into new markets, understanding the diverse and often less well-known legislative requirements can present difficulties. As the strategy lead for global pharmacovigilance of a leading rare diseases company highlighted, the Middle East and North Africa can be a difficult region to navigate because the expectations of the local authorities have been changing and even with an effort to create more harmonization in the region, the expectations and interpretations of each country can be slightly different.
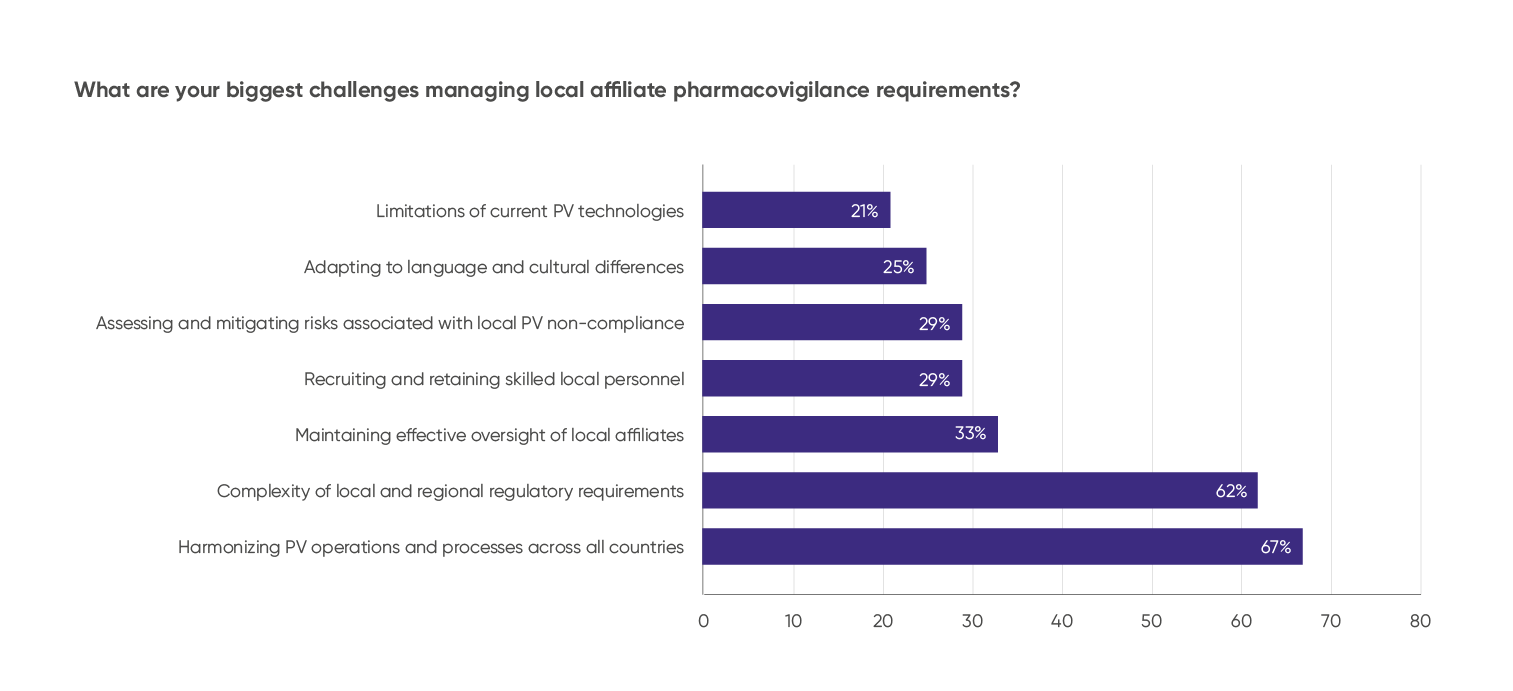
Complexity of local and regional regulations remains a pressing concern for many regulatory affairs and PV managers and country leads. This was reflected in a poll on the biggest challenges with managing local affiliate PV requirements, with a majority highlighting local complexities as their top concern, followed by harmonizing PV operations and processes across countries:
Source: Audience poll from PharmaLex webinar, Navigating Local Pharmacovigilance Obligations in a Global Landscape, held February 27, 2025.
Another PV challenge for companies is the reference submission application and how it aligns to the PV requirements of the countries being considered. As one speaker noted, if a reference submission application is from the US Food and Drug Administration but the country you are applying to is more aligned with Europe in terms of pharmacovigilance, that may create some issues when navigating that market’s legislative requirements.
Local interpretation
While health authorities are increasingly striving for harmonization, differences remain particularly with regard to how regulations are interpreted. The onus really is on companies to be more proactive in predicting what countries and regions expect and how those expectations are changing, one senior affiliate management expert from a large pharmaceutical company noted.
“Local experts have an important role to play in terms of sharing information, not only in terms of a review of the legislation but by communicating with the health authorities and gathering and sharing other local insights,” the expert noted.
Even in the European Union where there is a guideline on good pharmacovigilance practices (GVP) that applies to all countries and companies[1], there are differences in how GVP is interpreted, the PV partner and affiliate management lead for a large pharmaceutical company pointed out.
For companies that have out-licensed products to other companies in certain regions, there are the additional complexities of managing the expectations of partners.
Finding solutions to local PV challenges
As our experience shows, all these activities can be very resource-intensive and there is a scarcity of type of expertise needed at the local level. Some markets have well-developed regulations with strict requirements, but if the company’s activities in that market are low, it can be hard to find the right level of resource with the right background. Examples include Oman, which requires a qualified person for pharmacovigilance (QPPV) who is a pharmacist and based in the country to manage operational activities[2], and Colombia[3], which introduced new requirements recently, adding more complexity for companies unfamiliar with those expectations.
To overcome these challenges, many companies are turning to outsourcing partners to take on the day-to-day activities and have local resources focus on developing the strategy behind the launch of a product and risk minimization activities.
“When we launch a product in a new market, having external partners handle activities such as local literature searches and individual case safety report (ICSR) processing makes it much simpler for us from an internal resources point of view,” one of the speakers at the webinar noted.
Another speaker agreed, noting that outsourcing partners bring expertise from working with other companies and stakeholders to the relationship.
Communication and collaboration between global and local functions remains key. “Global needs to communicate what’s ongoing and what’s upcoming to the local functions so they are aware and can provide their feedback from a local perspective,” one of the speakers said. “Equally, we in global need to understand what’s happening at the affiliate level and any issues they might have. So regular communication to understand they are thinking is really key.”
That view was echoed by another speaker, who added: “If we don’t talk to each other, we can’t make sure the patients are kept safe. We need PV and other functions to let us know what they are planning in their daily business so we can prioritize patients.”
While understanding and adapting to diverse regulatory landscapes can pose significant challenges, ongoing and purposeful communication and collaboration between global and local teams, along with strategic outsourcing to experienced partners, can streamline compliance efforts and enhance patient safety. By embracing these approaches, companies can effectively manage local PV requirements and facilitate a successful market entry.
About the author:
José Miguel Rivas Romero is Program Manager, Service Line Lead for LPVS and Pharmacovigilance Expert, at PharmaLex. José Miguel has more than 10 years of experience in the pharmaceutical industry specializing in the design and implementation of local pharmacovigilance strategies across organizations.
[1] Good pharmacovigilance practices (GVP), European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/good-pharmacovigilance-practices-gvp
[2] Pharmacovigilance Systems in Arab Countries: Overview of 22 Arab Countries, Drug Saf. 2019. https://link.springer.com/article/10.1007/s40264-019-00807-4
[3] Invima Resolution 2024015321 of April 08, 2024. Invima Resolution 2024015321 of April 08, 2024 – Colombian Pharmacovigilance Association