Author: Nicole Tan
As described in the article, Addendum to TGA Deadlines: Medicine Labelling Compliance and COVID-19, 31 August 2020 marked the end of the medicines packaging reform transition. The intent of the reform was to make Australian medicine labels clearer and more informative for patients and consumers. As such, from 1 September 2020, all medicines released for supply must now comply with the relevant TGA labelling standard. Unless specifically excluded in the relevant Order (TGO 91 or TGO 92), it is illegal to supply medicines that are not compliant, unless prior consent has been provided by the TGA under section 14 of the Therapeutic Goods Act 1989.
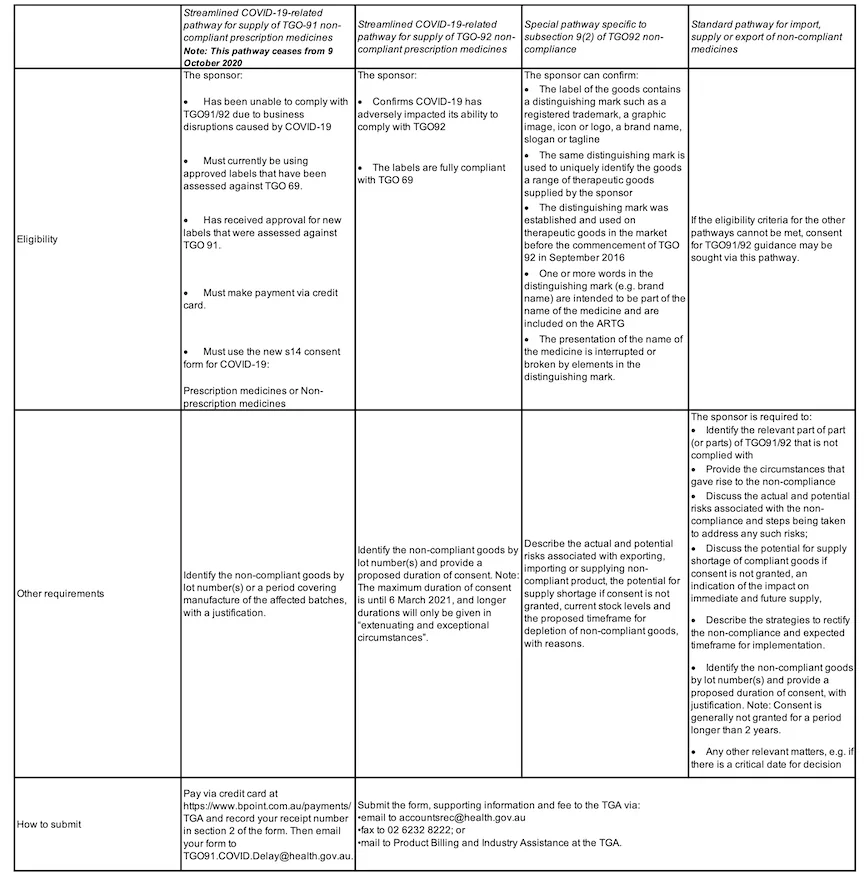
How do I claim an exemption?
A medicine sponsor can make an application for exemption to the TGA under the standard pathway, or via a temporary streamlined pathway that has been specifically designed to ease the business pressures that COVID-19 has placed on the industry, by making an allowance for delayed implementation of TGO91/92 compliant labels. To streamline this process, evaluation of product labelling is not required.
Please note the expedited pathway for section 14 consents for supply of medicines without TGO 91 compliant labels will cease on 9 October 2020.
In the addition to the above, there is also an interim process for non-prescription medicine sponsors to request consent for non-compliance with subsection 9(2) of TGO92, which requires that:
- the medicine name be presented in an uninterrupted and continuous manner (i.e. the complete name all in one place on the label).
- the use of trademarks, graphics or additional text must not disrupt the medicine name. This will ensure that the medicine name is clearly recognisable.
This option has been introduced to specifically address the difficulty that some sponsors have had in complying with this requirement because their brand name is iconic and includes a ‘distinguishing mark’ such as a registered trademark, a graphic image, logo, slogan or tagline, within or adjacent to the brand name.
What is the duration of consent?
Under each pathway, the sponsor will need to identify either impacted batch number(s) for the affected goods and/or nominate a period which would cover manufacture of the impacted batches. If using the latter approach, a rationale for the proposed duration of the period must be provided.
Note that all COVID-19-related consents to supply goods that do not comply with TGO92 will end on 6 March 2021, which aligns with the end of the transition period for permitted indications, a separate, but parallel reform also involving implementation of major label changes. In general, the duration of the consent from compliance with subsection 9(2) of TGO92 will be limited to 1 September 2021. A longer consent period for both exemption types may be considered under exceptional circumstances.
How much does an exemption cost?
The current cost is $490 per application. An application can include goods in multiple ARTG entries provided:
- the goods have the same sponsor
- the goods have the same non-compliance to the same part or parts of TGO91/92
- the goods have the same distinguishing mark, e.g. registered trademark, a graphic image, icon or logo, a brand name, slogan or tagline (only in the case of non-compliance with subsection 9(2) of TGO92)
How long does it take for TGA to decide on my exemption request?
A target of 5 working days for COVID-related TGO91 non-compliance, and
5-10 working days for COVID-19-related TGO92 non-compliance. A statutory or target timeframe for TGA’s decision has not been specified for the other pathways. Sponsors may also request priority review of a section 14 consent to allay the severity of a medicine shortage after submitting the relevant shortage notification. Any decision to reject must be notified to the sponsor within 28 days with reasons. In granting an exemption, the Delegate may impose a condition on the consent (under section 15 of the Act) to mitigate risk in supplying the non-compliant product.
Will the decision be made public?
Yes, all approved consents are publicly available on its website. The online repository permits searches by sponsor, product name, batch number, ARTG number, date of consent and year. Any decisions to reject are not required to be published. Consents granted via two of the pathways (standard and TGO91 streamlined) are published on TGA’s website:
- Database of consents to import, supply or export therapeutic goods that do not comply with standards
- Database of consents for prescription medicines that do not comply with TGO 91 labelling due to COVID-19
Can I appeal the decision?
Yes, the rights of review are included in the decision letter to the sponsor. The sponsor has a right under section 60 of the Therapeutic Goods Act 1989 to seek an internal review of the TGA’s decision or the condition(s) imposed on consent within 90 days of being notified. The sponsor may also request a review by the Administrative Appeals Tribunal within 28 days starting on the day the decision was made.
Which pathway should I apply under and what are the data requirements?
This may depend on the particular circumstances of the impacted product and/or the reason for the delay in implementation. Table 1 describes the eligibility criteria for the respective pathways.
Table 1 Summary of the pathways available to sponsors of medicines which may be non-compliant with the applicable labelling orders