Would you travel to your holiday location in a plane that fulfills all its technical requirements — it has wheels, it has wings, it has engines, and also fuel! — or in a plane that has been demonstrated to take-off, fly, and land without issues with high probability?
At PharmaLex we don’t know much about planes, but we do know a lot about pharma topics. And in pharma a similar analogy holds. We are sure that you rather take a tablet released on the market using an analytical method that has fulfilled all regulatory expectations but also has demonstrated to provide results of high accuracy with high probability.
The question now is how can the (bio)pharmaceutical producers simultaneously provide reliable analytical results and be compliant to regulations?
Since the adoption of the ICH Q8 document concerning the development of pharmaceutical processes following a quality by design (QbD) approach, there have been many discussions on whether and how the analytical procedure development should follow a similar approach. Authorities such as US Pharmacopoeia and ICH are currently preparing tomorrows’ regulations to implement Analytical QbD and introduce the idea of Analytical or Assay Life Cycle. Evidence of this is shown by the implementation of new guidance (see Table 1): revision of ICH Q2 for the validation of analytical methods, introduction of the ICH M10 for the validation of Bioanalytical Methods, the future implementation of ICH Q14 for analytical quality by design as well as the numerous proposals of chapters in the USP regarding fitness for use of assays, uncertainty of measurements, analytical target profile or even reportable values. And then there are these brand-new documents such as USP 1210 chapter for proper statistical assessment of validation results and the USP 1032 and 1033 ones for development and validation of bioassays. A new document is also available as a draft about the Analytical Procedure Life Cycle (USP 1220).
How can PharmaLex be of help? PharmaLex software, Enoval, and Seelva, generate reports for the qualification or validation of either physico-chemical or biological assays, while Transval is software that assesses if a method can be transferred from a “sender” lab to one or several “receiver” labs.
They fulfill the current and trends in regulations for two key steps of the Life Cycle of Analytical Methods and Bioassays, namely Validation (Performance Qualification) and Transfer. This software is fully validated (GAMP5) and 21 CFR part 11 compliant and as such ready to be used within GxP environments.
All three solutions allow you to obtain fully compliant reports in few minutes and implement the proposals of USP 1210 chapter by using statistical tolerance intervals and total error to decide if the analytical methods are fit for their purpose. At the end of these studies, not only will you have trust in your analytical methods or bioassays but much more: You will gain trust in the analytical results obtained using them.
Above all, the knowledge about the reliability of your analytical results at the end of the method’s validation or transfer is perfectly known based on the Accuracy Profile (aka Total Error Profile) β-expectation tolerance interval. Indeed, knowing that the analytical method has good performance is important (remember: it got wheels, it got wings!), but even more the knowledge that every, single, future analytical result will be accurate and thus reliable enough in order to make the correct decisions. With such knowledge, releasing a new batch can be made with more trust and confidence.
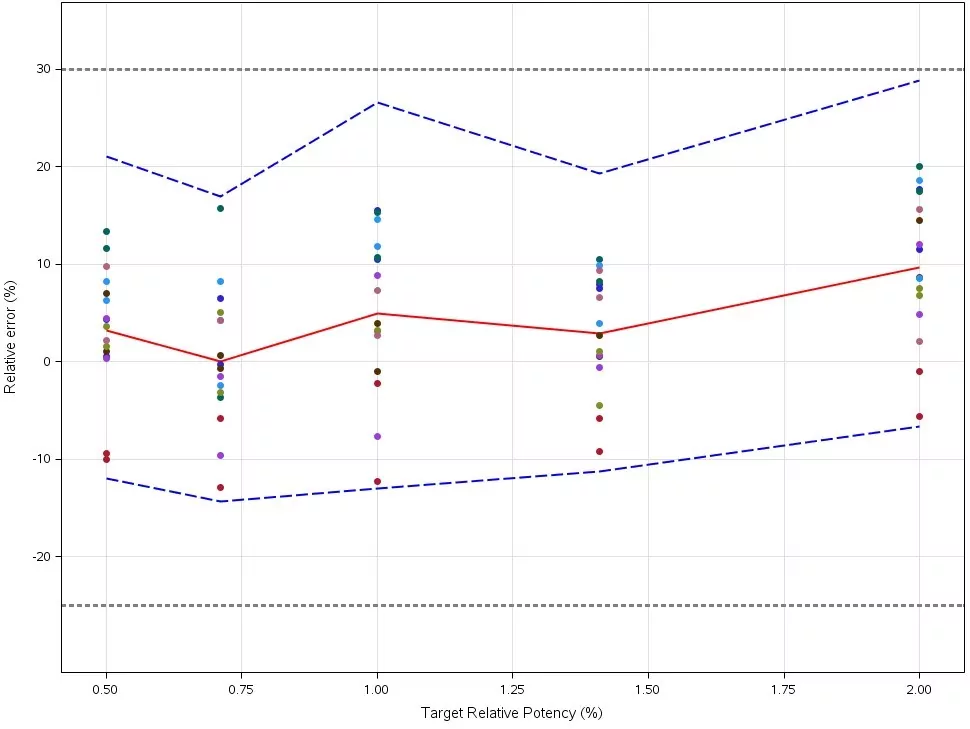
So how does it work? The accuracy profile (aka total error profile) shown in Figure 1 conveys in a simple way to scientists as well as authorities the level of reliability of the results that will be produced by the bioassay or analytical method. The black dotted line is the predefined 30 % acceptance criteria and the blue dotted line is the 95 % tolerance interval showing that there is less than a 5 % chance for future results to fall outside the acceptance criteria.
Figure 1: Accuracy Profile (aka total error profile) obtained for the validation of a parallel curve Bioassay. Dashed blue lines: 95% β-expectation tolerance intervals; Dotted black lines: Acceptance limits expressed as Total Error (%); Colored dots: results of the validation samples expressed in relative error (%).
These approaches implemented in our software have been used by our customers since 2003. Indeed, our software and experienced consultants have been supporting you in implementing these upcoming regulations for nearly two decades! Concretely: implementing the adequate statistical strategy to develop a regulatory compliant and useful method validation or transfer, as well as helping in adapting your internal SOPs and quality documentation. An advantage you may not find easily elsewhere.
Table 1: List of regulatory guidance documents fulfilled by Enoval, Seelva & Transval. NA: Not
Applicable; *: Draft documents or under discussion
| (Bio)Pharmaceutical | Enoval | Seelva | Transval |
| ICH Q2(R1): Validation of analytical procedures: text and methodology | ✓ | ✓ | ✓ |
| ICH Q14: Analytical Procedure Lifecycle Management* | ✓ | ✓ | ✓ |
| FDA-2015: Analytical Procedures and Methods Validation for Drugs and Biologics | ✓ | ✓ | ✓ |
| USP 1225: Validation of Compendial Methods | ✓ | ✓ | ✓ |
| USP 1224: Transfer of Analytical Procedures | NA | NA | ✓ |
| USP 1210: Statistical Tools for Procedure Validation | ✓ | ✓ | ✓ |
| USP 1033: Biological Assay Validation | NA | ✓ | NA |
| USP 1220: Analytical Procedure Life Cycle* | ✓ | ✓ | ✓ |
| Bioanalysis | |||
| ICH M10: Bioanalytical Method Validation* | ✓ | ✓ | ✓ |
| FDA-2018: Guidance for Industry: Bioanalytical Method Validation | ✓ | ✓ | ✓ |
| EMA2011: Guideline on bioanalytical method validation | ✓ | ✓ | ✓ |
| Veterinary | |||
| VICH GL1 & GL2 | ✓ | ✓ | NA |