Author: Luis Jimenez
Australia’s, Therapeutic Goods Administration (TGA) issued a notice on 1 October 2020, in addition to an email to stakeholders prior, specifying new policies for the inclusion of non-sterile, non-measuring Class 1 Medical Devices. These changes include:
- Enhancements to the application for inclusion in the Australian Register of Therapeutic Goods (ARTG) form; and
- Modification of the process by which the TGA reviews applications for inclusion in the ARTG.
Types of Class I Medical Devices exempt from these changes
The TGA noted that the changes will not include Class I IVD medical devices, Class I Export Only and Class I IVD Export Only devices.
Changes to existing Class I Medical Device ARTG entries
The notification did not include any changes to already existing Class I Medical Devices.
Targeted Improvements
With these changes, the TGA aims to make it easier for sponsors to provide the required information for TGA to make a registration decision and reduce the need to ask for more information.
What is changing
- All ARTG applications of Class I Medical Devices will be required to submit an Australian Declaration of Conformity. Sponsors have always been required to hold a DOC on file, but this will now be required to be submitted as part of the application. The TGA has also revised this form to be more user friendly.
- The change to the assessment process will help the TGA confirm if the product is a medical device, that it is correctly classified, that the device has the appropriate procedures applied to it and that the information is complete and correct.
What is not changing
- The change on the process will not change the typical approval time of one (1) day. In some cases, it may take up to four (4) business days or longer if selected for an audit.
What TGA’s Declaration of Conformity (DOC) entails
Templates for Declaration of Conformity are found in at the TGA’s website and they vary by classification and conformity assessment route. The DOC for Sponsors of Class I Medical Devices requires the following:
- Manufacturers Name
- Business Address
- Classification Type
- GMDN Code and term
- Standards Applied to the Device
- Name of Medical Devices.
- Most importantly, they have to provide a confirmation that the Manufacturer meets the Essential Principles and has the Technical Documentation to back it up.
Until now, Class I registrations did not need supplemental application forms other than the ARTG application.
Some Background on Class I Registrations in Australia
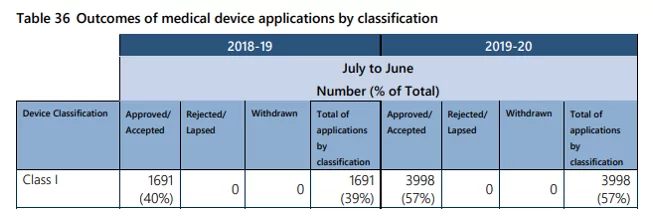
According to the latest TGA Annual performance statistics report issued this month Class I registrations increased from 39% to 57% of the total number of medical device ARTG applications (see table 36 from the report). The change aims to streamline this process.
Historically reviews of Class I application were conducted by restricted word reviews which the TGA described as follows:
“Class I medical devices are included on the ARTG following a self-certification being made online by the sponsor through a computer-generated decision process, we undertake post-market compliance reviews for these devices. This previously included restricted word reviews (up until January 2020), where potentially inappropriate Class I device inclusions are identified by the use of specific words indicative of risk, or listing issues relating to the inclusion of the device.
Our current practice is to assess all new Class I ARTG inclusions and request information where there may be uncertainty regarding the appropriateness of the inclusion.”
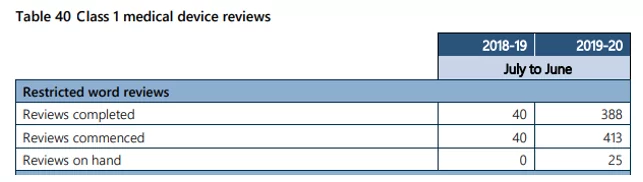
The report indicated that approximately 413 reviews were triggered this FY compared to 40 in the previous year (see Table 40 below).
Conclusion
The change will help the TGA confirm various factors important to determine if a manufacturer has the appropriate data and is the classification is correct for Class I Medical Device and the anticipated impact of the review is of up to 4 business days, so it should not impact manufacturers much while providing TGA with the ability to better regulate this Class of Medical Devices.
If you have any questions regarding these changes, please reach out, we have a staff of specialised regulatory experts keeping up to date to the current and evolving regulatory environment with regards to the TGA and all major regulatory bodies.
References
TGA Announcement of Changes:
https://www.tga.gov.au/changes-artg-inclusion-process-non-measuring-non-sterile-class-i-medical-devices-0
2020 TGA Annual Statistical Report: https://www.tga.gov.au/sites/default/files/annual-performance-statistics-report-july-2019-june-2020.pdf
Declaration of Conformity Templates:
https://www.tga.gov.au/form/declaration-conformity-templates-medical-devicesContact us








