Author: Luis Jimenez
Marking the second year after the issuance of the revised TGA Advertising framework, the TGA has evidenced a strong hard-line position on enforcements against the illegal import, supply, and advertising of therapeutic goods into Australia during the fiscal year for 2019-2020 (starting in July).
During the twelve months, 187 infringement notices were issued totalling almost $1.8 million in fines. The TGA also pursued civil court cases against three companies.
In direct response to COVID-19 advertising, there were 58 infringement notices (representing 31% of all notices for the fiscal year) totalling $549 thousand in fines. The COVID-19 related false advertisement trend has continued in August and TGA will likely continue to carefully monitor any advertisements involving COVID-19 to ensure the safety of the public. In some instances, this could even include social media, as was seen in a widely publicised case in which an Australian media character with 1.4 million subscribers was issued a hefty fine as a result of a Facebook live stream where unsupported COVID-19 related claims were made.
As with most regulators the array of actions that the TGA can take include several avenues depending on the magnitude of the violation:
- Warning letters
- Suspensions
- Cancellations
- Recall actions
- Advertising directions notices
- Infringement notices
- Enforceable undertakings
- Injunctions
- Civil penalties
- Criminal prosecutions
- Advertising complaints outcomes
- Listed medicine compliance review outcomes
The TGA calculates the amount payable for an infringement notice using the formula in the Act (subsection 42YKA(3)). Below is a representation of the formula:
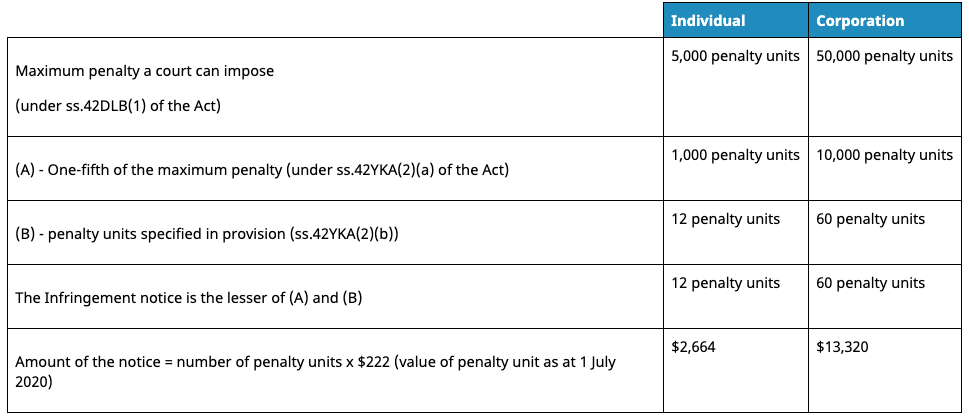
In respect to the total amounts, the TGA state:
“We do not have discretion to set the value of the infringement notice even if we are of the view the particular alleged offence may warrant a higher amount.”
An independent assessment of the new Advertisement Framework:
On June 2020, the TGA published the independent report announced in 2018 by Australia’s Minister of Health to examine the impact of the Therapeutic Goods advertisement framework. Amongst other items including educational programs of the industry, the revised framework includes:
- Increased clarity and objectivity to support the compliance and enforcement powers in the Act and improved consistency between the requirements for medicines and medical devices;
- Appointed the TGA as the single body responsible for implementing a complaints management process about the advertising of therapeutic goods to the public; and
- Broadened sanctions and penalties to deter inappropriate and misleading advertising of therapeutic goods.
The assessment evaluated activities over the 12-18 month period after the implementation of the changes to the advertisement framework, namely 2018 to February 2020. The final report on the impact of advertising reforms from the Expert Panel Review outlined a total of 22 recommendations, which were all supported by the Government. Here is an overall overview of the TGA’s key takeaways and plans.
“The agreed actions, once implemented, will deliver improvements and refinements to the therapeutic goods advertising framework, including:
- the development, in consultation with stakeholders, of annual advertising compliance priorities, for outcomes and consumer-focused approach to advertising compliance;
- implementation of more strategic advertising complaints handling that uses complaints as a source of intelligence, to be used for individual case selection and in determining compliance priorities;
- the development and publication of new key performance indicators focused on priorities and outcomes rather than processes and deadlines;
- the publication of case studies based on case experience to assist advertisers and consumers to understand the legal requirements for advertising therapeutic goods, in conjunction with the ongoing publication of policy clarification as needed;
- improved flexibility, so as to be adaptable to emerging issues, and improved transparency through media and publications, as modelled on our response to compliance during the COVID-19 pandemic;
- implementation of an education and stakeholder engagement strategy, aligned with compliance priorities and supported by education priorities, which focuses on consumer and industry benefit;
- enhanced collaboration and engagement with other Australian regulators; and
- enhancing the operation of the Therapeutic Goods Advertising Consultative Committee.”
Use of the Advertisement Framework in response to COVID-1
“Since the analysis included the initial months of the COVID-19 environment, it provided an opportunity for the TGA to demonstrate the value to consumers of focusing on compliance priorities, taking a strategic approach to communication and education tasks, and adopting an agile approach to the use of its enhanced powers.”
The report refrained from making specific recommendations about the COVID-19 related response, but provide some insight into the mindset of the TGA indicating.
“In consultations conducted towards the end of the review, Departmental staff indicated that there had been an internal shift in attitude, towards much more hardline enforcement in response to the more serious risk atmosphere presented by misleading advertising around COVID-19 claims. In recent times, there has also been increased media coverage of inappropriate and misleading COVID-19 related therapeutic goods advertising, and the fines that have been handed out. This has been supported by increased use of multiple public notices, particularly to warn consumers about illegal advertising about COVID-19. The Departmental staff comments, a high-level overview of information from the compliance database, and the media coverage of recent cases indicate that there has been an increase in the timely use of stronger enforcement responses, such as fines and public notices, with regard to COVID-related advertising.
The COVID-19 experience also has useful learnings regarding the use of media and publicising the compliance actions taken by the TGA to raise awareness of the negative consequences of using non-compliant advertising.”
Conclusion
TGA seem to strike a good balance on active, intelligent, and alert monitoring of breaches to the advertisement code. Seemingly, the main areas of concern of social media and use of the media to advertise are being effectively employed by the TGA to educate and motivate the industry to stay compliant. The use of media to publicise fines and attract media attention to those that breach the code, maybe in many cases, more harm than the actual fines.
The new advertisement framework provides requirements for any advertisement and marketing activity in Australia. Please reach out to us if you have questions regarding the compliance of your advertisement activities in Australia.
References:
Main article source published by TGA on July 8th 2020: https://www.tga.gov.au/media-release/tga-issues-fines-nearly-18-million-alleged-illegal-activity-2019-20
Report for Review of Therapeutic Goods Advertising Framework-August 10th 2020:
https://www.tga.gov.au/sites/default/files/review-therapeutic-goods-advertising-framework.pdf
Australian Government response to the Review of the Reforms to the Therapeutic Goods Advertising Framework-August 10th 2020: https://www.tga.gov.au/australian-government-response-review-reforms-therapeutic-goods-advertising-framework
TGA issues fines of nearly $1.8 million for alleged illegal activity in 2019-20:
https://www.tga.gov.au/media-release/tga-issues-fines-nearly-18-million-alleged-illegal-activity-2019-20
We tailor our services to the requirements of your business and can help you implement plans that are best suited to your projects. Marking the second year after the issuance of the revised TGA Advertising framework, the TGA has evidenced a strong hard-line position on enforcements against the illegal import, supply, and advertising of therapeutic goods into Australia during the fiscal year for 2019-2020 (starting in July).
During the twelve months, 187 infringement notices were issued totalling almost $1.8 million in fines. The TGA also pursued civil court cases against three companies.
In direct response to COVID-19 advertising, there were 58 infringement notices (representing 31% of all notices for the fiscal year) totalling $549 thousand in fines. The COVID-19 related false advertisement trend has continued in August and TGA will likely continue to carefully monitor any advertisements involving COVID-19 to ensure the safety of the public. In some instances, this could even include social media, as was seen in a widely publicised case in which an Australian media character with 1.4 million subscribers was issued a hefty fine as a result of a Facebook live stream where unsupported COVID-19 related claims were made.
As with most regulators the array of actions that the TGA can take include several avenues depending on the magnitude of the violation:
- Warning letters
- Suspensions
- Cancellations
- Recall actions
- Advertising directions notices
- Infringement notices
- Enforceable undertakings
- Injunctions
- Civil penalties
- Criminal prosecutions
- Advertising complaints outcomes
- Listed medicine compliance review outcomes
The TGA calculates the amount payable for an infringement notice using the formula in the Act (subsection 42YKA(3)). Below is a representation of the formula:

In respect to the total amounts, the TGA state:
“We do not have discretion to set the value of the infringement notice even if we are of the view the particular alleged offence may warrant a higher amount.”
For a description of details on what each action entails and lists of recent enforcement actions on each click here.
An independent assessment of the new Advertisement Framework:
On June 2020, the TGA published the independent report announced in 2018 by Australia’s Minister of Health to examine the impact of the Therapeutic Goods advertisement framework. Amongst other items including educational programs of the industry, the revised framework includes:
- Increased clarity and objectivity to support the compliance and enforcement powers in the Act and improved consistency between the requirements for medicines and medical devices;
- Appointed the TGA as the single body responsible for implementing a complaints management process about the advertising of therapeutic goods to the public; and
- Broadened sanctions and penalties to deter inappropriate and misleading advertising of therapeutic goods.
The assessment evaluated activities over the 12-18 month period after the implementation of the changes to the advertisement framework, namely 2018 to February 2020. The final report on the impact of advertising reforms from the Expert Panel Review outlined a total of 22 recommendations, which were all supported by the Government. Here is an overall overview of the TGA’s key takeaways and plans.
“The agreed actions, once implemented, will deliver improvements and refinements to the therapeutic goods advertising framework, including:
- the development, in consultation with stakeholders, of annual advertising compliance priorities, for outcomes and consumer-focused approach to advertising compliance;
- implementation of more strategic advertising complaints handling that uses complaints as a source of intelligence, to be used for individual case selection and in determining compliance priorities;
- the development and publication of new key performance indicators focused on priorities and outcomes rather than processes and deadlines;
- the publication of case studies based on case experience to assist advertisers and consumers to understand the legal requirements for advertising therapeutic goods, in conjunction with the ongoing publication of policy clarification as needed;
- improved flexibility, so as to be adaptable to emerging issues, and improved transparency through media and publications, as modelled on our response to compliance during the COVID-19 pandemic;
- implementation of an education and stakeholder engagement strategy, aligned with compliance priorities and supported by education priorities, which focuses on consumer and industry benefit;
- enhanced collaboration and engagement with other Australian regulators; and
- enhancing the operation of the Therapeutic Goods Advertising Consultative Committee.”
Use of the Advertisement Framework in response to COVID-1
“Since the analysis included the initial months of the COVID-19 environment, it provided an opportunity for the TGA to demonstrate the value to consumers of focusing on compliance priorities, taking a strategic approach to communication and education tasks, and adopting an agile approach to the use of its enhanced powers.”
The report refrained from making specific recommendations about the COVID-19 related response, but provide some insight into the mindset of the TGA indicating.
“In consultations conducted towards the end of the review, Departmental staff indicated that there had been an internal shift in attitude, towards much more hardline enforcement in response to the more serious risk atmosphere presented by misleading advertising around COVID-19 claims. In recent times, there has also been increased media coverage of inappropriate and misleading COVID-19 related therapeutic goods advertising, and the fines that have been handed out. This has been supported by increased use of multiple public notices, particularly to warn consumers about illegal advertising about COVID-19. The Departmental staff comments, a high-level overview of information from the compliance database, and the media coverage of recent cases indicate that there has been an increase in the timely use of stronger enforcement responses, such as fines and public notices, with regard to COVID-related advertising.
The COVID-19 experience also has useful learnings regarding the use of media and publicising the compliance actions taken by the TGA to raise awareness of the negative consequences of using non-compliant advertising.”
Conclusion
TGA seem to strike a good balance on active, intelligent, and alert monitoring of breaches to the advertisement code. Seemingly, the main areas of concern of social media and use of the media to advertise are being effectively employed by the TGA to educate and motivate the industry to stay compliant. The use of media to publicise fines and attract media attention to those that breach the code, maybe in many cases, more harm than the actual fines.
The new advertisement framework provides requirements for any advertisement and marketing activity in Australia. Please reach out to us if you have questions regarding the compliance of your advertisement activities in Australia.
References:
Main article source published by TGA on July 8th 2020: https://www.tga.gov.au/media-release/tga-issues-fines-nearly-18-million-alleged-illegal-activity-2019-20
Report for Review of Therapeutic Goods Advertising Framework-August 10th 2020:
https://www.tga.gov.au/sites/default/files/review-therapeutic-goods-advertising-framework.pdf
Australian Government response to the Review of the Reforms to the Therapeutic Goods Advertising Framework-August 10th 2020: https://www.tga.gov.au/australian-government-response-review-reforms-therapeutic-goods-advertising-framework
TGA issues fines of nearly $1.8 million for alleged illegal activity in 2019-20:
https://www.tga.gov.au/media-release/tga-issues-fines-nearly-18-million-alleged-illegal-activity-2019-20
We tailor our services to the requirements of your business and can help you implement plans that are best suited to your projects. Contact us