Governments across the globe are starting to re-open, Society and people are returning to their workplaces. So, what does this mean for the future of virtual audits? Were they a phenomenon of the pandemic or did the pandemic teach us that by developing technology enabled solutions to complement the technical skillset of our employees, it is possible that virtual audits can continue to provide a real alternative to on-site audits?
An interesting conundrum given the emphasis that is being put on sustainability, reducing carbon footprint and employees ‘new norm’ of operating in a virtual environment.
Let’s investigate…..
As we know, it is imperative for companies to engage in proactive auditing to ensure their supply cain management is in a constant state of compliance and is sufficiently robust to be able to manage unforeseen circumstances. It is incumbent upon companies to ensure their suppliers and contracted organisations continue to supply goods and services that are fit for purpose, meet the required GxP standard and to maintain the terms of quality and technical agreements.
So, what are the key questions to be considered to determine whether to conduct a virtual or on-site audit?
Risk Management Plan
Pharmaceutical companies can improve the success of both virtual and on-site audits by developing a well-considered risk management plan and strategy for both virtual and on-site audits.
Firstly, create a risk management plan which scopes out your entire supply change. Once the risk assessment determines the requirement to carry out an audit, the next step is to determine the most appropriate type of audit: on-Site or virtual.
Once the audit is completed, consideration should be given to the next audit required and the type selected. There may be cases where the audit findings may indicate that an onsite audit should be completed the next time. This may be due to expressions of concern or due to difficulty assessing a particular area by virtual means.
The risk assessment should also be updated with information on quality considerations such as quality defects, customer complaints as they arise. This information may change the audit type selected. This risk management plan should be updated accordingly and reviewed periodically to ensure an accurate assessment remains.
In addition to standard considerations when conducting an on-site audit, when considering virtual auditing, the following considerations will help determine the success of your Virtual Audit Programme:
ICT:
How robust is the Auditee’s ICT? Does it allow for the transfer of data guaranteeing reliable information security and protection of confidentiality? How adequate is the Wi-Fi or broadband access to allow for a smooth auditing process?
Has the Auditee an eQMS Document Management System or do they operate a paperbased QMS? Some smaller suppliers may not have the capital available to invest in an eQMS Document Management System. If paper-based, what steps would be required to assist the Auditee to transfer this information through a safe cloud solution?
The Auditor
How proficient is the Auditor in the use of ICT? Do they have access to a suitable remote work environment that minimizes interruptions, noise, and other distractions?
Quality of Objective Evidence
As we have previous outlined in our recent Virtual Audit Whitepaper, another challenge is ensuring the quality of objective evidence. As we know, the purpose of conducting an audit is to evaluate the adequacy of the supplier’s operation and their compliance with GxP requirements. For this assessment to be comprehensive and to stand up to regulatory scrutiny, the ability to gather sufficient objective evidence is of paramount importance.
Setting your Virtual Audit up for success:
To help companies to implement successful virtual audits, we recommend consideration of the following to allow for effective sharing of such evidence with auditors whilst maintaining security and confidentiality:
- Can the audit include real-time images?
- Can interviews and feedback be recorded?
- How can the company ensure that the auditor receives an objective and thorough view of the facility, equipment, operations, and controls?
- How can the company provide access to relevant information without compromising privacy or confidentiality?
- What type of file sharing is appropriate, and what access levels are needed?
- Is the company’s cloud storage plan sufficient for document transfer?
- Which documents can be viewed via camera or online, and which need to be delivered via courier?
Resolving these challenges is a key part of planning an effective virtual audit.
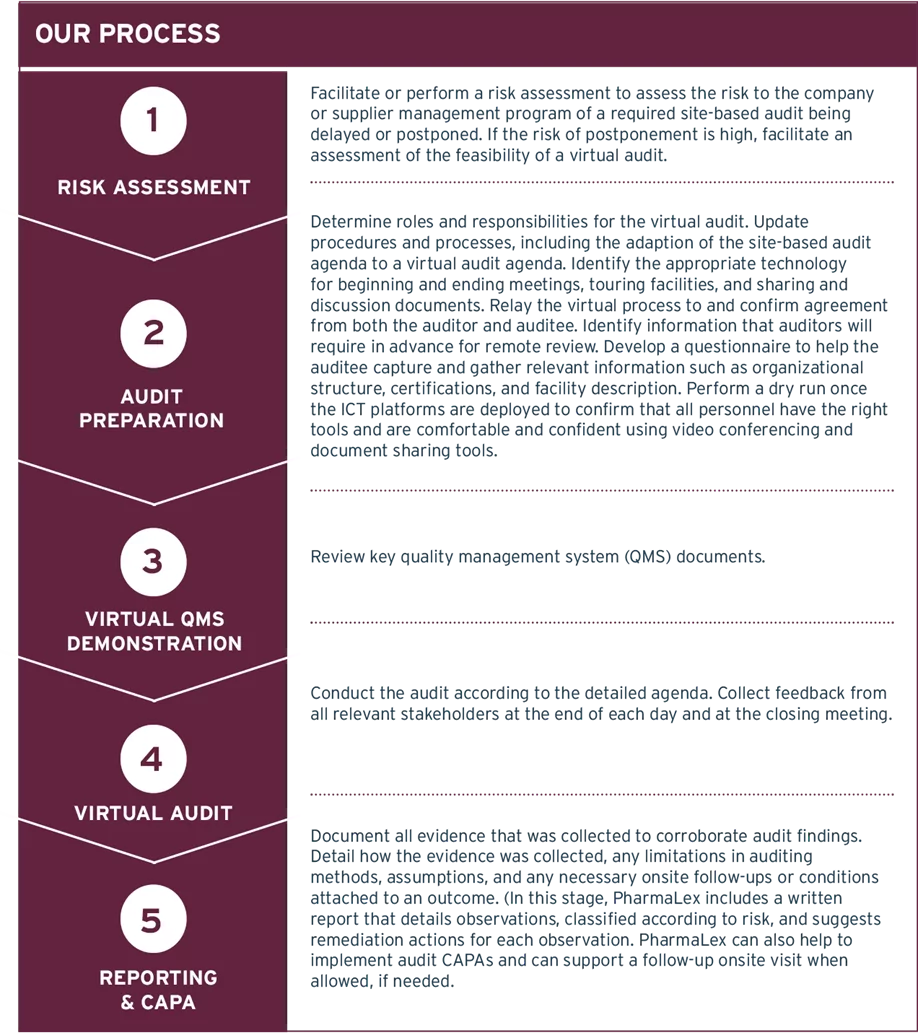
To help pharmaceutical companies implement successful remote audits, PharmaLex has developed a virtual auditing model that combines these elements into an efficient 5-stage workflow, as illustrated in Figure 1.
Virtual Audits to Remain
Even though virtual audits became a necessity during the covid-19 pandemic, most pharmaceutical companies now realise they are an integral way of making an assessment of a potential or current outsourced activity provider. It is however important to remember to use them in conjunction with robust justification, always ensuring adequate assessment is completed and switching to onsite audits in higher risk situations.
If you require any assistance with keeping your audit program on track, please do not hesitate to contact us here