WHAT IS A SIGNAL
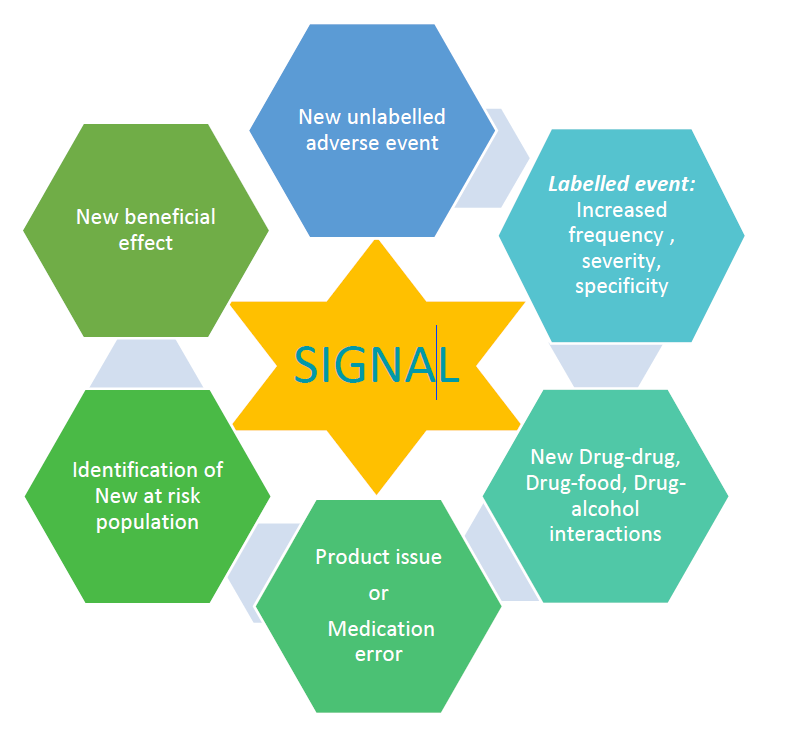
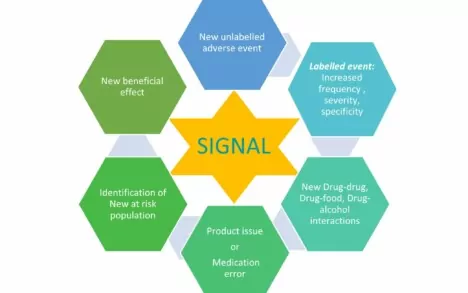
A signal can be an increase in frequency/severity/specificity of a labelled event or a new unlabelled adverse event or a newly identified drug-drug/drug-food/drug-alcohol interactions or a medication error/product issue or identification of a new population at risk or a new beneficial effect of a drug
Information that arises from one or multiple sources (including observations and experiments), which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action. (CIOMS VIII – Practical Aspects of Signal Detection in Pharmacovigilance)
A concern about an excess of adverse events compared to what would be expected to be associated with a product’s use. (FDA)
Reported information on a possible causal relationship between an adverse event and a drug, the relationship being unknown or incompletely documented previously. (WHO)
A signal suggests a possible causal association between the drug and the adverse event.
WHY DO WE NEED TO PERFORM SIGNAL DETECTION?
Any new medicinal product prior to getting a marketing approval from any regulatory authority would have to show positive benefit-risk ratio (i.e., the anticipated benefits for the intended indication should outweigh the risks or harmful effects from the use of the medicinal product/intervention). The information on this benefit-risk is reached at, through years of experimenting and researching in the laboratories, animals, and humans through pre-clinical and clinical studies (Phase I-IV). At the time of marketing approval, the product would have shown positive benefit-risk ratio based on the human exposure in the clinical trials (often limited to a few thousand).
The limited exposure in the clinical trials and with exclusion criteria’s (primarily excluding paediatric, elderly, pregnant & lactating woman, renal & hepatic impaired, medical histories, etc.), lack of long-term treatment effect, and limited concomitant medication exposure, the clinical trials do not reach the power to identify all the adverse reactions (rare/very rare) possible with a drug. Hence, there is limited information on the safety & efficacy of a medicinal product at the time of receiving marketing approval
To ensure the benefit-risk ratio of a product always stays positive, the Marketing authorisation holders (MAHs) are expected to perform continuous reviews of all the possible sources to identify new safety information related to a product. The knowledge on the drug’s safety profile, pharmacological actions, rare adverse events, and clinical effectiveness increases over the years following approval. This is usually achieved by active surveillance by expedited reporting, periodic reports, and signal detection activities with mutual exchange of safety data with the regulators. Hence, we perform signal detection.
WHAT ARE THE SOURCES OF SIGNALS
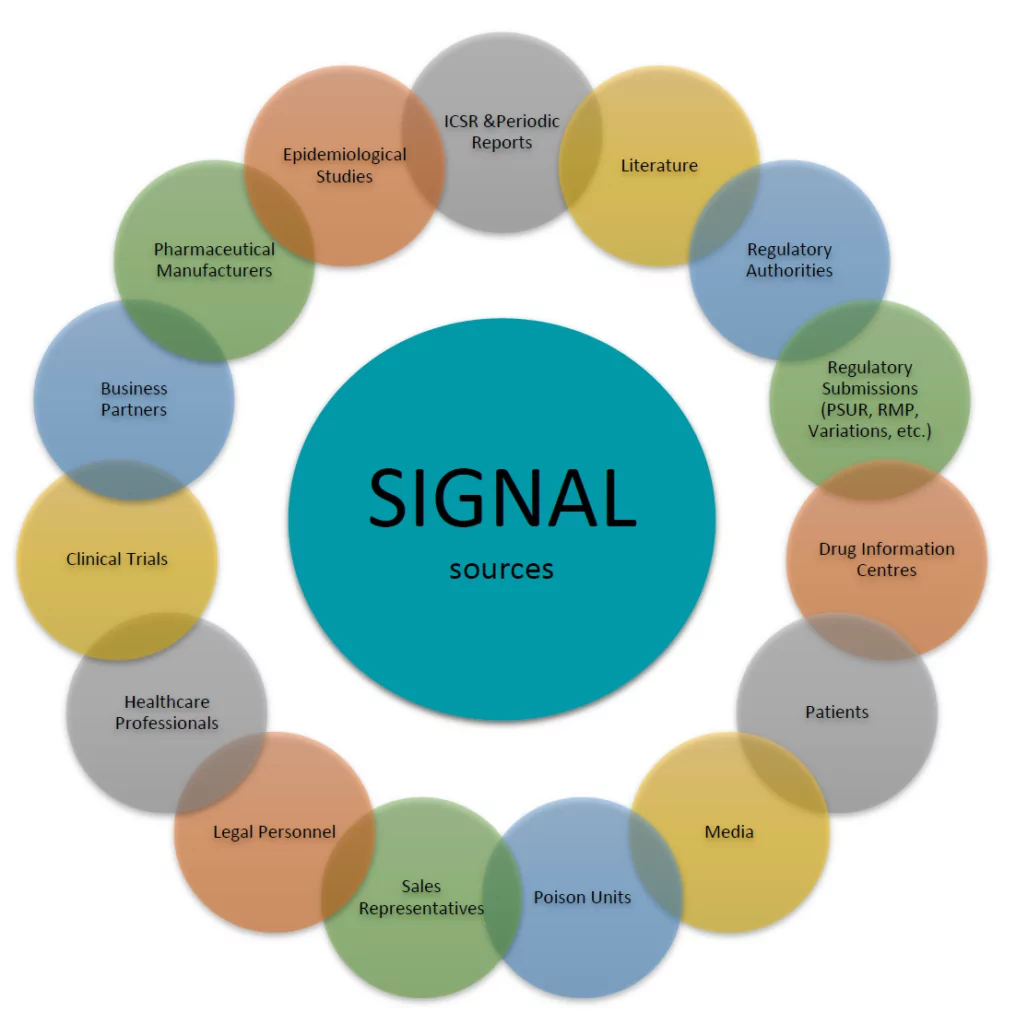
A signal can arise from multiple sources as depicted in the figure below.
WHAT ARE THE CHALLENGES IN SIGNAL DETECTION?
The road to detecting, validating and confirming signals is usually filled with potholes making the journey of identifying, evaluating and establishing a signal/risk, sometimes very challenging.
- Limited information on the background incidence of the event in the untreated general population
- Lack of information on the frequency of the events in the drug exposed population (considering the deficiencies in the reported cases/events)
- The reporting frequency and the number of reported cases is less for drugs with less exposure
- Limited information on the temporal association, dose-effect, medical history, co-morbid conditions, and concomitant medications
- Effects of long-term exposure or effects in the long-term following exposure (e.g. Exposure to diethylsilbesterol [DES] in pregnant women and development of vaginal carcinoma in their daughters)
HOW DOES PHARMALEX HELP OVERCOME THESE CHALLENGES
Our team of experts are well versed and experienced in evaluating and critically appraising ICSRs, aggregate data, and literature and thereby providing a comprehensive evaluation report considering the strengths and weaknesses of all the data sets and sources reviewed.
Where data on a safety issue is limited, our experts customize a search strategy which is not only specific but also sensitive which addresses the medical topic.
As needed, our safety experts liaise with our toxicology experts in critically appraising and evaluating the pre-clinical and toxicology data to compliment and to provide a comprehensive evaluation of the safety issue.Contact us