ICSR Management
The collection and processing of ICSRs are the most basic and important aspects of Pharmacovigilance system. PharmaLex delivers end-to-end ICSR Management solutions through our experts and services.
Contact our specialists to tailor a service plan
ICSR (Individual Safety Case Report) Management
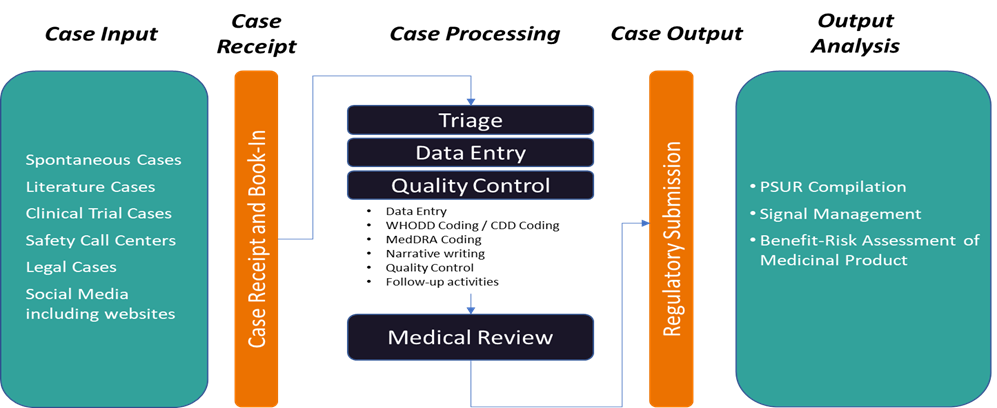
PharmaLex offer the full range of ICSR Management Services from collection, triage, Data Entry ,Quality Review and Medical Review for regulatory submission of ICSRs.PharmaLex provide Safety Case Management services in various therapeutics areas and products in Pharmaceuticals, Radiopharmaceuticals, Nutraceuticals, Cosmetovigilance, Materiovigilance, Biologics / Biosimilars etc. :
We offer global solutions covering the entire product lifecycle
Our solutions, consisting of core services, which are tailored to address each of your unique requirements. Extensive program management expertise is a key success factor within our service solutions.
End-to-end Safety Case management
Individual Case Study Report (ICSR) plays an important role as it acts as a source of data in pharmacovigilance processes and activities.
Cases Reconciliation
Reconciliation of safety cases is performed as defined in Pharmacovigilance Agreements and contracts.
PharmaLex perform safety cases reconciliations with:
- Company affiliates
- Partners
- Medical Information department
- Product Complaints team
- Clinical Trials
We ensure that the reconciliation process is performed between MAH and above-mentioned parties in a periodic manner or as defined in the respective PVAs (Pharmacovigilance Agreements), Safety Management Plans and other contracts.
Shaping The Future
Automation, artificial intelligence (AI) and machine learning are revolutionizing many industries enabling increased productivity, improved quality and enhancing return on investment. The pharmaceutical industry is still largely relying on established, but increasingly outdated, systems and methodologies that were efficient many years ago yet now prove to be a limiting factor in innovation and growth.
At PharmaLex we combine our extensive global experience and deep understanding of pharmaceutical lifecycle processes with the development of our cloud-based platform for life sciences automation alongside key partnerships with leading technology vendors. Through our pharma technology-enabled services we are helping our customers to adopt and take advantage of innovative technologies to drive efficiency gains within their own operations.
At PharmaLex we have the technology, know-how and experience to take these raw ingredients and realize real, immediate, and lasting benefits for our clients – we call this SmartPHLEX.
- SmartVigiscreen – managing EVWEB download cases: SmartVigiScreen is a cloud-based software solution that processes incoming ICSRs automating the analysis of company vs non-company cases and reducing screening workload significantly.
GxP validated system according to GAMP 5 guidelines and CFR Title 21 Part 11 compliant.
- SmartVigicontract – Efficient management of PV contracts: SmartVigicontract is a cloud-based software solution for pharmacovigilance teams who need to handle large amounts of data deriving from contracts, such as Pharmacovigilance Agreements (PVAs) and Safety Data Exchange Agreements (SDEAs). The SmartVigicontract application serves as a single trusted source of information for generation of PVA overviews.
Safety Database: At PharmaLex our expert team in technology, consulting and operations support the entire pharmacovigilance journey including safety systems management, safety database migrations, etc.
- PharmaLex has rich experience of working on multiple pharmacovigilance databases including those of our clients. Our processors have experience processing cases on variety of safety database, standard systems like Argus/ARISg/AB Cube / Veeva / PVNet / Basecon / BPI Pheda etc. and also on custom built in house proprietary safety databases.
- For many clients, PharmaLex provide safety database solution. It has a fully validated E2B(R2/R3) compatible safety database for an end to end case processing services solution. For such customers, PharmaLex is completely responsible for the database management including provision of database, vendor management, computer system validation and change management, maintenance of configured settings (e.g. users, products), maintenance of related procedural documents. PharmaLex’ R&D Informatics team supports these activities, as well as database related user enquiries.
- Our Safety database services also includes comprehensive support during the migration process from planning to quality control. PharmaLex holds scalable resources to perform (semi)manual migrations or conducts automated migrations together with database providers.
- Safety databases are provided as part of PharmaLex’ service landscape including the provision of technology, vendor management, computer system validation and change management, maintenance of configured settings (e.g. users, products), maintenance of related procedural documents.
- Our technology services include database provisioning, database management, advanced safety data reporting, analytics and insights.
Available Resources
- 3rd August 2021
FURTHER RESOURCES
Related Services
Clinical Trials Adverse Event Processing
Clinical trials play a crucial part in any drug development life cycle where it is key to gain information on a product’s safety profile. The PharmaLex international team of clinical safety experts, with large expierence in the management of clinical safety activities, effectively supports sponsors with Pharmacovigilance activities during clinical trial development programmes. PharmaLex designs […]
More InfoGap Analysis and Consulting
Place your Pharmacovigilance, Epidemiology, Risk management (PER) and Document Management activities in experienced hands. Whether you need an urgent solution for an unexpected problem or are seeking to outsource entire processes – our knowledgeable experts will be there for you no matter where you are. Expertise extends to Clinical document management (e.g. TMFs) and Regulatory document management (e.g. submissions, RIM, xEVMPD, IDMP) through one of the PharmaLex family of companies.
More InfoLiterature Surveillance
We offer a modular, end-to-end approach to literature surveillance for pharmacovigilance and our experts are flexible enough to offer a tailored solution as well accordingly to client’s requirement.
More InfoMonitoring and Signal Detection
PharmaLex customizes the signal management activities as per client requirements ensuring reliable, high quality, trackable data in compliance with the regulatory guidelines (e.g., GVP Module IX & FDA Good Pharmacovigilance Practice and Pharmacoepidemiologic Assessment). In addition to Signal management services, our team of experts are also experienced and well versed in providing responses to safety questions from the Health authorities by providing strategic advice on the approach to respond to the HA query; evaluating and critically appraising ICSRs, aggregate data, and literature and thereby providing a comprehensive evaluation report considering the strengths and weaknesses of all the data sets and sources reviewed.
More InfoPharmacovigilance Audits
PharmaLex’s organization ensures global delivery of all PV audit services. We are the first-choice provider of PV audit services for many pharmaceutical companies around the world. At PharmaLex, auditors are highly qualified with necessary and long-standing expierence as well as strong cultural awareness and excellent communication skills to effectively conduct and/or participate in PV audit activities.
More InfoQualified Person for Pharmacovigilance (QPPV)
If required, PharmaLex can take on the tasks and responsibilities of the EU Qualified Person Responsible for Pharmacovigilance, or provide a deputy in times of absence or illness.
More InfoContact Us
Contact Form
Complete this form to stay in contact with us.
Global Approach
PharmaLex has 60+ offices in 32 countries serving more than 1600 satisfied clients worldwide. Search your nearest office.