Author: Luis Jimenez
This article is current as at 29 April 2020
The European Parliament has voted to postpone the implementation deadline for MDR from May 2020 to May 2021. This gives a 12-month extension for those device manufacturers who have gotten caught in the middle. For our analysis on the extension click here.
Now that companies have a choice of MDD or MDR, and many companies are looking for emergency use reviews for their products, we cover who you can you go to for European registrations and provide corresponding contact information.
MDR ((EU) 2017/745) Certified Notified Bodies:
There is a total of 13 Notified Bodies certified for MDR. Interestingly, many of the already certified notified bodies have had revisions to their certifications. See below table including the latest MDR certification date:
| Notified Body Number | Notified Body (and link) | Country | Date of latest MDR Certification |
| 0086 | BSI Assurance UK Ltd | United Kingdom | 13/02/2020 |
| 2797 | BSI Group The Netherlands B.V. | Netherlands | 07/02/2020 |
| 2409 | CE Certiso Orvos- és Kórháztechnikai Ellenőrző | Hungary | 21/03/2020 |
| 1912 | DARE!! Services B.V. | Netherlands | 5/11/2019 |
| 0344 | DEKRA Certification B.V. | Netherlands | 06/03/2020 |
| 0124 | DEKRA Certification GmbH | Germany | 28/08/2019 |
| 2460 | DNV GL Presafe AS | Norway | 06/02/2020 |
| 0051 | IMQ ISTITUTO ITALIANO DEL MARCHIO DI QUALITÀ S.P.A. | Italy | 20/08/2019 |
| 0483 | MDC MEDICAL DEVICE CERTIFICATION GMBH | Germany | 25/04/2020 |
| 0482 | MEDCERT ZERTIFIZIERUNGS- UND PRÜFUNGSGESELLSCHAFT FÜR DIE MEDIZIN GMBH | Germany | 25/12/2019 |
| 0050 | National Standards Authority of Ireland (NSAI) | Ireland | 13/02/2020 |
| 0197 | TÜV Rheinland LGA Products GmbH | Germany | 26/09/2019 |
| 0123 | TÜV SÜD Product Service GmbH Zertifizierstellen | Germany | 06/11/2019 |
IVDR ((EU) 2017/746) Notified Bodies:
Below is a table identifying the three notified bodies that are currently IVDR certified.
| Notified Body Number | Notified Body (and link) | Country | Date of latest IVDR Certification |
| 0086 | BSI Assurance UK Ltd | United Kingdom | 13/02/2020 |
| 2797 | BSI Group The Netherlands B.V. | Netherlands | 07/02/2020 |
| 0124 | DEKRA Certification GmbH | Germany | 10/10/2019 |
| 0086 | BSI Assurance UK Ltd | United Kingdom | 13/02/2020 |
Pending Notified Bodies:
Although not extensive, this is a list of the notified bodies that have issued statements of their intention to apply for MDR and/or IVDR certification.
| Notified Body Number | Notified Body (and link) | MDR | IVDR |
| 0633 | Berlin Cert Prüf- und Zertifizierstelle für Medizinprodukte GmbH | Yes | Unknown |
| 0297 | DQS Medizinprodukte GmbH | Yes | Unknown |
| 1282 | ENTE CERTIFICAZIONE MACCHINE SRL | Yes | Unknown |
| 0413 | INTERTEK SEMKO AB | Yes | Unknown |
| 1434 | POLSKIE CENTRUM BADAN I CERTYFIKACJI S.A. | Yes | Yes |
| TBD | QMD Services | Yes | Yes |
| 1639 | SGS Belgium NV | Yes | Yes |
| 0120 | SGS United Kingdom Limited | Yes | Yes |
| 0044 | TÜV NORD CERT GmbH | Yes | Unknown |
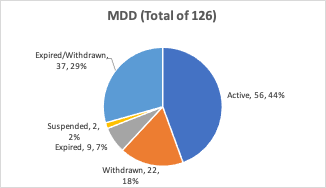
MDD (93/42/EEC MDD) Certified Notified Bodies:
Now that the MDR deadline has been moved 12-months and companies may be looking for an MDD certificate, below is the current distribution of MDD certified Notified Bodies:
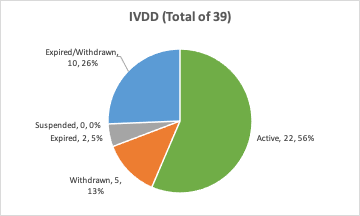
IVDD (98/79/EC IVD) Certified Notified Bodies:
The IVDR implementation deadline is not due until 2022, so IVDD certifications are still very much in demand. Below is the distribution of total IVDD notified bodies:
Conclusions
In comparison to the original total number of MDD certified Notified Bodies of 126, only 13 have current MDR certification. This reflects how rushed the MDR transition was even before the COVID-19 Pandemic, given that the original deadline was scheduled for May 2020. Further for the IVDR, the number of Notified Bodies for IVDs is reflecting a similar lag from 39 original IVDD notified bodies to 3 IVDR certified bodies. To date, for both IVD and Medical Device Notified Bodies, about 10% of them have made the cut to certification under the new regulations.
The MDR extension is sure going to help. In terms of IVDs, Med Tech Europe has already started to request that the IVDR be delayed (Click here).
Overall, these transitions are never easy, but they are manageable. When the TGA transitioned to its version of IVDR some 5 years ago, there was certainly a lot of pain, and then the dust settled, and we got on our way. We expect the same to happen in Europe, albeit on a bigger scale and with much larger global implications.
Let’s not forget that CE Mark has been historically the passport for approvals in a majority of international markets.
Need Help?
Navigating the ever-changing world of Notified Bodies can be perplexing. If you are having issues, let us help. Reach out to initiate a conversation.
References:
European Commission Website: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.main
Useful Link to Queries:
MDR Certified Notified Bodies: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=34
IVDR Certified Notified Bodies: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=35
MDD Certified Notified Bodies: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=13
IVD D Certified Notified Bodies: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=20Contact us








